Human myeloperoxidase (hMPO) is expressed in neurons in the substantia nigra in Parkinson's disease and in the hMPO-α-synuclein-A53T mouse model, correlating with increased nitration and aggregation of α-synuclein and exacerbation of motor impairment
- PMID: 31175983
- PMCID: PMC6774439
- DOI: 10.1016/j.freeradbiomed.2019.05.033
Human myeloperoxidase (hMPO) is expressed in neurons in the substantia nigra in Parkinson's disease and in the hMPO-α-synuclein-A53T mouse model, correlating with increased nitration and aggregation of α-synuclein and exacerbation of motor impairment
Abstract
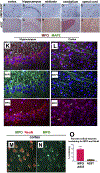
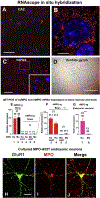
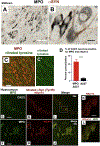
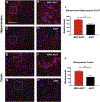
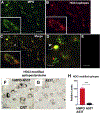
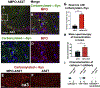
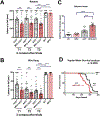
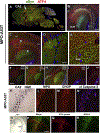
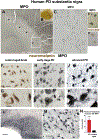
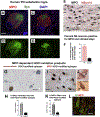
α-Synuclein (αSyn) is central to the neuropathology of Parkinson's disease (PD) due to its propensity for misfolding and aggregation into neurotoxic oligomers. Nitration/oxidation of αSyn leads to dityrosine crosslinking and aggregation. Myeloperoxidase (MPO) is an oxidant-generating enzyme implicated in neurodegenerative diseases. In the present work we have examined the impact of MPO in PD through analysis of postmortem PD brain and in a novel animal model in which we crossed a transgenic mouse expressing the human MPO (hMPO) gene to a mouse expressing human αSyn-A53T mutant (A53T) (hMPO-A53T). Surprisingly, our results show that in PD substantia nigra, the hMPO gene is expressed in neurons containing aggregates of nitrated αSyn as well as MPO-generated HOCl-modified epitopes. In our hMPO-A53T mouse model, we also saw hMPO expression in neurons but not mouse MPO. In the mouse model, hMPO was expressed in neurons colocalizing with nitrated αSyn, carbamylated lysine, nitrotyrosine, as well as HOCl-modified epitopes/proteins. RNAscope in situ hybridization confirmed hMPO mRNA expression in neurons. Interestingly, the hMPO protein expressed in hMPO-A53T brain is primarily the precursor proMPO, which enters the secretory pathway potentially resulting in interneuronal transmission of MPO and oxidative species. Importantly, the hMPO-A53T mouse model, when compared to the A53T model, exhibited significant exacerbation of motor impairment on rotating rods, balance beams, and wire hang tests. Further, hMPO expression in the A53T model resulted in earlier onset of end stage paralysis. Interestingly, there was a high concentration of αSyn aggregates in the stratum lacunosum moleculare of hippocampal CA2 region, which has been associated in humans with accumulation of αSyn pathology and neural atrophy in dementia with Lewy bodies. This accumulation of αSyn aggregates in CA2 was associated with markers of endoplasmic reticulum (ER) stress and the unfolded protein response with expression of activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), MPO, and cleaved caspase-3. Together these findings suggest that MPO plays an important role in nitrative and oxidative damage that contributes to αSyn pathology in synucleinopathies.
Keywords: Alpha synuclein; Carbamylation; Dementia with lewy bodies; ER stress; Hippocampus CA2 region; Hypochlorous acid; MPO-H(2)O(2)-chloride system; Myeloperoxidase; Nitration; Parkinson's disease; Reactive oxygen species; Synucleinopathies; Unfolded protein response.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Thiocyanate Reduces Motor Impairment in the hMPO-A53T PD Mouse Model While Reducing MPO-Oxidation of Alpha Synuclein in Enlarged LYVE1/AQP4 Positive Periventricular Glymphatic Vessels.Antioxidants (Basel). 2022 Nov 26;11(12):2342. doi: 10.3390/antiox11122342. Antioxidants (Basel). 2022. PMID: 36552550 Free PMC article.
-
Alpha-synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity.Acta Neuropathol. 2019 Jun;137(6):939-960. doi: 10.1007/s00401-019-01993-2. Epub 2019 Mar 15. Acta Neuropathol. 2019. PMID: 30877431 Free PMC article.
-
Mutant α-Synuclein Overexpression Induces Stressless Pacemaking in Vagal Motoneurons at Risk in Parkinson's Disease.J Neurosci. 2017 Jan 4;37(1):47-57. doi: 10.1523/JNEUROSCI.1079-16.2016. J Neurosci. 2017. PMID: 28053029 Free PMC article.
-
Neuropathology of α-synuclein in Parkinson's disease.Neuropathology. 2022 Apr;42(2):93-103. doi: 10.1111/neup.12812. Epub 2022 Mar 31. Neuropathology. 2022. PMID: 35362115 Review.
-
α-Synuclein Strains and Their Relevance to Parkinson's Disease, Multiple System Atrophy, and Dementia with Lewy Bodies.Int J Mol Sci. 2023 Jul 28;24(15):12134. doi: 10.3390/ijms241512134. Int J Mol Sci. 2023. PMID: 37569510 Free PMC article. Review.
Cited by
-
Energy, Entropy and Quantum Tunneling of Protons and Electrons in Brain Mitochondria: Relation to Mitochondrial Impairment in Aging-Related Human Brain Diseases and Therapeutic Measures.Biomedicines. 2021 Feb 22;9(2):225. doi: 10.3390/biomedicines9020225. Biomedicines. 2021. PMID: 33671585 Free PMC article. Review.
-
The Roles of Neutrophil-Derived Myeloperoxidase (MPO) in Diseases: The New Progress.Antioxidants (Basel). 2024 Jan 22;13(1):132. doi: 10.3390/antiox13010132. Antioxidants (Basel). 2024. PMID: 38275657 Free PMC article. Review.
-
MPO/HOCl Facilitates Apoptosis and Ferroptosis in the SOD1G93A Motor Neuron of Amyotrophic Lateral Sclerosis.Oxid Med Cell Longev. 2022 Feb 7;2022:8217663. doi: 10.1155/2022/8217663. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35178161 Free PMC article.
-
Nutraceuticals Targeting Generation and Oxidant Activity of Peroxynitrite May Aid Prevention and Control of Parkinson's Disease.Int J Mol Sci. 2020 May 21;21(10):3624. doi: 10.3390/ijms21103624. Int J Mol Sci. 2020. PMID: 32455532 Free PMC article. Review.
-
Thiocyanate Reduces Motor Impairment in the hMPO-A53T PD Mouse Model While Reducing MPO-Oxidation of Alpha Synuclein in Enlarged LYVE1/AQP4 Positive Periventricular Glymphatic Vessels.Antioxidants (Basel). 2022 Nov 26;11(12):2342. doi: 10.3390/antiox11122342. Antioxidants (Basel). 2022. PMID: 36552550 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

