Targeting ADP-ribosylation as an antimicrobial strategy
- PMID: 31176616
- PMCID: PMC7172630
- DOI: 10.1016/j.bcp.2019.06.001
Targeting ADP-ribosylation as an antimicrobial strategy
Abstract
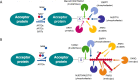
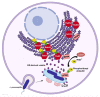
ADP-ribosylation (ADPr) is an ancient reversible modification of cellular macromolecules controlling major biological processes as diverse as DNA damage repair, transcriptional regulation, intracellular transport, immune and stress responses, cell survival and proliferation. Furthermore, enzymatic reactions of ADPr are central in the pathogenesis of many human diseases, including infectious conditions. By providing a review of ADPr signalling in bacterial systems, we highlight the relevance of this chemical modification in the pathogenesis of human diseases depending on host-pathogen interactions. The post-antibiotic era has raised the need to find alternative approaches to antibiotic administration, as major pathogens becoming resistant to antibiotics. An in-depth understanding of ADPr reactions provides the rationale for designing novel antimicrobial strategies for treatment of infectious diseases. In addition, the understanding of mechanisms of ADPr by bacterial virulence factors offers important hints to improve our knowledge on cellular processes regulated by eukaryotic homologous enzymes, which are often involved in the pathogenesis of human diseases.
Keywords: ADP-ribosyl transferase (ART); ADP-ribosylation; ART bacterial toxins; Antimicrobial strategies; Poly(ADP-ribose) polymerase (PARP).
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict or financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

