Maternal Multiple Micronutrient Supplementation Stabilizes Mitochondrial DNA Copy Number in Pregnant Women in Lombok, Indonesia
- PMID: 31177276
- PMCID: PMC6686057
- DOI: 10.1093/jn/nxz064
Maternal Multiple Micronutrient Supplementation Stabilizes Mitochondrial DNA Copy Number in Pregnant Women in Lombok, Indonesia
Abstract
Background: The Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) in Lombok, Indonesia showed that maternal multiple micronutrients (MMN), as compared with iron and folic acid (IFA), reduced fetal loss, early infant mortality, and low birth weight. Mitochondria play a key role during pregnancy by providing maternal metabolic energy for fetal development, but the effects of maternal supplementation during pregnancy on mitochondria are not fully understood.
Objective: The aim of this study was to assess the impact of MMN supplementation on maternal mitochondrial DNA copy number (mtDNA-CN).
Methods: We used archived venous blood specimens from pregnant women enrolled in the SUMMIT study. SUMMIT was a cluster-randomized double-blind controlled trial in which midwives were randomly assigned to distribute MMN or IFA to pregnant women. In this study, we selected 108 sets of paired baseline and postsupplementation samples (MMN = 54 and IFA = 54). Maternal mtDNA-CN was determined by real-time quantitative polymerase chain reaction in baseline and postsupplementation specimens. The association between supplementation type and change in mtDNA-CN was performed using rank-based estimation for linear models.
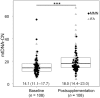
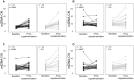
Results: In both groups, maternal mtDNA-CN at postsupplementation was significantly elevated compared with baseline (P < 0.001). The regression revealed that the MMN group had lower postsupplementation mtDNA-CN than the IFA group (β = -4.63, P = 0.003), especially for women with mtDNA-CN levels above the median at baseline (β = -7.49, P = 0.007). This effect was rapid, and observed within 33 d of initiation of supplementation (β = -7.39, P = 0.017).
Conclusion: Maternal MMN supplementation rapidly stabilized mtDNA-CN in pregnant women who participated in SUMMIT, indicating improved mitochondrial efficiency. The data provide a mechanistic basis for the beneficial effects of MMN on fetal growth and survival, and support the transition from routine IFA to MMN supplementation.This trial was registered at www.isrctn.com as ISRCTN34151616.
Keywords: iron and folic acid; mitochondrial DNA copy number; mtDNA-CN; multiple micronutrients; oxidative stress; pregnancy; supplementation.
Copyright © American Society for Nutrition 2019.
Figures
Comment in
-
Antenatal Micronutrients and the Mitochondrial Genome: A Glimpse of Future Nutritional Investigation.J Nutr. 2019 Aug 1;149(8):1303-1304. doi: 10.1093/jn/nxz101. J Nutr. 2019. PMID: 31177285 No abstract available.
References
-
- Gittelsohn J, Thapa M, Landman LT. Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Soc Sci Med. 1997;44:1739–49. - PubMed
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J; Maternal and Child Undernutrition Study Group. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. - PubMed
-
- Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group, Shankar AH, Jahari AB, Sebayang SK, Aditiawarman, Apriatni M, Harefa B, Muadz H, Soesbandoro SDA, Tjiong R et al. .. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet. 2008;371:215–27. - PubMed
-
- Roberfroid D, Huybregts L, Lanou H, Habicht J-P, Henry M-C, Meda N, Kolsteren P. Prenatal micronutrient supplements cumulatively increase fetal growth. J Nutr. 2012;142:548–54. - PubMed

