6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial
- PMID: 31178152
- PMCID: PMC6615016
- DOI: 10.1016/S0140-6736(19)30650-6
6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial
Abstract
Background: Adjuvant trastuzumab significantly improves outcomes for patients with HER2-positive early breast cancer. The standard treatment duration is 12 months but shorter treatment could provide similar efficacy while reducing toxicities and cost. We aimed to investigate whether 6-month adjuvant trastuzumab treatment is non-inferior to the standard 12-month treatment regarding disease-free survival.
Methods: This study is an open-label, randomised phase 3 non-inferiority trial. Patients were recruited from 152 centres in the UK. We randomly assigned patients with HER2-positive early breast cancer, aged 18 years or older, and with a clear indication for chemotherapy, by a computerised minimisation process (1:1), to receive either 6-month or 12-month trastuzumab delivered every 3 weeks intravenously (loading dose of 8 mg/kg followed by maintenance doses of 6 mg/kg) or subcutaneously (600 mg), given in combination with chemotherapy (concurrently or sequentially). The primary endpoint was disease-free survival, analysed by intention to treat, with a non-inferiority margin of 3% for 4-year disease-free survival. Safety was analysed in all patients who received trastuzumab. This trial is registered with EudraCT (number 2006-007018-39), ISRCTN (number 52968807), and ClinicalTrials.gov (number NCT00712140).
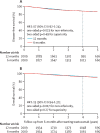
Findings: Between Oct 4, 2007, and July 31, 2015, 2045 patients were assigned to 12-month trastuzumab treatment and 2044 to 6-month treatment (one patient was excluded because they were double randomised). Median follow-up was 5·4 years (IQR 3·6-6·7) for both treatment groups, during which a disease-free survival event occurred in 265 (13%) of 2043 patients in the 6-month group and 247 (12%) of 2045 patients in the 12-month group. 4-year disease-free survival was 89·4% (95% CI 87·9-90·7) in the 6-month group and 89·8% (88·3-91·1) in the 12-month group (hazard ratio 1·07 [90% CI 0·93-1·24], non-inferiority p=0·011), showing non-inferiority of the 6-month treatment. 6-month trastuzumab treatment resulted in fewer patients reporting severe adverse events (373 [19%] of 1939 patients vs 459 [24%] of 1894 patients, p=0·0002) or stopping early because of cardiotoxicity (61 [3%] of 1939 patients vs 146 [8%] of 1894 patients, p<0·0001).
Interpretation: We have shown that 6-month trastuzumab treatment is non-inferior to 12-month treatment in patients with HER2-positive early breast cancer, with less cardiotoxicity and fewer severe adverse events. These results support consideration of reduced duration trastuzumab for women at similar risk of recurrence as to those included in the trial.
Funding: UK National Institute for Health Research, Health Technology Assessment Programme.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures



Comment in
-
Is the duration of adjuvant trastuzumab debate still clinically relevant?Lancet. 2019 Jun 29;393(10191):2565-2567. doi: 10.1016/S0140-6736(19)30946-8. Epub 2019 Jun 6. Lancet. 2019. PMID: 31178153 No abstract available.
-
PERSEPHONE - implications for clinical practice in 2019.Nat Rev Clin Oncol. 2019 Nov;16(11):663-664. doi: 10.1038/s41571-019-0256-7. Nat Rev Clin Oncol. 2019. PMID: 31341271 No abstract available.
-
Protective strategies to prevent trastuzumab-induced cardiotoxicity.Lancet. 2020 Feb 15;395(10223):491-492. doi: 10.1016/S0140-6736(19)32549-8. Lancet. 2020. PMID: 32061289 No abstract available.
References
-
- Slamon DJ, Leyland-Jones B, Shak S. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–92. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–72. - PubMed
-
- Romond EH, Perez EA, Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–84. - PubMed
-
- Cameron D, Piccart-Gebhart MJ, Gelber RD. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. - PMC - PubMed
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017; 389: 1195–205. - PMC - PubMed
Uncited Reference
-
- Curtis C, Shah SP, Chin SF. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. - PMC - PubMed
- Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486: 346–52. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

