Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials
- PMID: 31178289
- PMCID: PMC6595176
- DOI: 10.1016/S1473-3099(19)30126-4
Efficacy of live oral rotavirus vaccines by duration of follow-up: a meta-regression of randomised controlled trials
Abstract
Background: The duration of protection offered by rotavirus vaccines varies across the world, and this variation is important to understanding and predicting the effects of the vaccines. There is now a large body of evidence on the efficacy of live oral rotavirus vaccines in different settings, but these data have never been synthesised to obtain robust estimates of efficacy by duration of follow-up. Our aim is to estimate the efficacy of live oral rotavirus vaccines at each point during follow-up and by mortality stratum.
Methods: In our meta-regression study, we identified all randomised controlled trials of rotavirus vaccines published until April 4, 2018, using the results of a Cochrane systematic review, and cross checked these studies against those identified by another systematic review. We excluded trials that were based on special populations, trials without an infant schedule, and trials without clear reporting of numbers of enrolled infants and events in different periods of follow-up. For all reported periods of follow-up, we extracted the mean duration of follow-up (time since administration of the final dose of rotavirus vaccination), the number of enrolled infants, and case counts for rotavirus-positive severe gastroenteritis in both non-vaccinated and vaccinated groups. We used a Bayesian hierarchical Poisson meta-regression model to estimate the pooled cumulative vaccine efficacy (VE) and its waning with time for three mortality strata. We then converted these VE estimates into instantaneous VE (iVE).
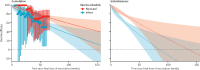
Findings: In settings with low mortality (15 observations), iVE pooled for infant schedules of Rotarix and RotaTeq was 98% (95% credibility interval 93-100) 2 weeks following the final dose of vaccination and 94% (87-98) after 12 months. In medium-mortality settings (11 observations), equivalent estimates were 82% (74-92) after 2 weeks and 77% (67-84) after 12 months. In settings with high mortality (24 observations), there were five different vaccines with observation points for infant schedules. The pooled iVE was 66% (48-81) after 2 weeks of follow-up and 44% (27-59) after 12 months.
Interpretation: Rotavirus vaccine efficacy is lower and wanes more rapidly in high-mortality settings than in low-mortality settings, but the earlier peak age of disease in high-mortality settings means that live oral rotavirus vaccines are still likely to provide substantial benefit.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Rotavirus vaccine protection in low-income and middle-income countries.Lancet Infect Dis. 2019 Jul;19(7):673-674. doi: 10.1016/S1473-3099(19)30263-4. Epub 2019 Jun 6. Lancet Infect Dis. 2019. PMID: 31178290 Free PMC article. No abstract available.
-
Efficacy of live oral rotavirus vaccines.Lancet Infect Dis. 2019 Sep;19(9):929. doi: 10.1016/S1473-3099(19)30428-1. Lancet Infect Dis. 2019. PMID: 31478512 No abstract available.
References
-
- GBD Diarrhoeal Diseases Collaborators Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909–948. - PMC - PubMed
- GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017; 17: 909–48. - PMC - PubMed
-
- Lanata CF, Fischer-Walker CL, Olascoaga AC. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8 - PMC - PubMed
- Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8: e72788. - PMC - PubMed
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62(suppl 2):S96–S105. - PMC - PubMed
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance Network. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62 (suppl 2): S96–105. - PMC - PubMed
-
- Rheingans RD, Antil L, Dreibelbis R, Podewils LJ, Bresee JS, Parashar UD. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis. 2009;200(suppl 1):S16–S27. - PubMed
- Rheingans RD, Antil L, Dreibelbis R, Podewils LJ, Bresee JS, Parashar UD. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis 2009; 200 (suppl 1): S16–27. - PubMed
-
- Thomas SL, Walker JL, Fenty J. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35:680–686. - PMC - PubMed
- Thomas SL, Walker JL, Fenty J, et al. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine 2017; 35: 680–86. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

