Repositioning Glucagon Action in the Physiology and Pharmacology of Diabetes
- PMID: 31178432
- PMCID: PMC7085250
- DOI: 10.2337/dbi19-0004
Repositioning Glucagon Action in the Physiology and Pharmacology of Diabetes
Abstract
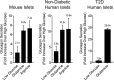
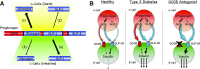
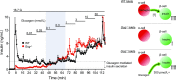
Glucagon is historically described as the counterregulatory hormone to insulin, induced by fasting/hypoglycemia to raise blood glucose through action mediated in the liver. However, it is becoming clear that the biology of glucagon is much more complex and extends beyond hepatic actions to exert control on glucose metabolism. We discuss the inconsistencies with the canonical view that glucagon is primarily a hyperglycemic agent driven by fasting/hypoglycemia and highlight the recent advances that have reshaped the metabolic role of glucagon. These concepts are placed within the context of both normal physiology and the pathophysiology of disease and then extended to discuss emerging strategies that incorporate glucagon agonism in the pharmacology of treating diabetes.
© 2019 by the American Diabetes Association.
Figures

References
-
- Gosmanov NR, Gosmanov AR, Gerich JE. Glucagon physiology. In Endotext. Feingold KR, Anawalt B, Boyce A, et al., Eds. South Dartmouth, MA, 2000
-
- MacCuish AC, Munro JF, Duncan LJ. Treatment of hypoglycaemic coma with glucagon, intravenous dextrose, and mannitol infusion in a hundred diabetics. Lancet 1970;2:946–949 - PubMed
-
- Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

