Hydrogen Sulfide Attenuates High Glucose-Induced Human Retinal Pigment Epithelial Cell Inflammation by Inhibiting ROS Formation and NLRP3 Inflammasome Activation
- PMID: 31178664
- PMCID: PMC6507269
- DOI: 10.1155/2019/8908960
Hydrogen Sulfide Attenuates High Glucose-Induced Human Retinal Pigment Epithelial Cell Inflammation by Inhibiting ROS Formation and NLRP3 Inflammasome Activation
Abstract
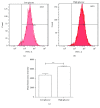
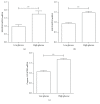
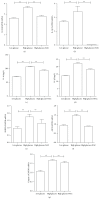
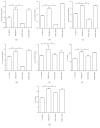
Hydrogen sulfide (H2S) has been shown to protect against oxidative stress injury and inflammation in various high glucose-induced insult models. However, it remains unknown whether H2S protects human retinal pigment epithelial cells (RPE cells) from high glucose-induced damage. In the current study, cell viability, proinflammatory cytokines, ROS, and inflammasome markers were compared in a low glucose- and high glucose-induced cell culture system. The antioxidant N-acetylcysteine (NAC), NLRP3 siRNA, and NaHS were used to test RPE cell responses. The results demonstrate that compared with the low-glucose culture, high glucose triggered higher cell death and increased IL-18 and IL-1β mRNA expression and protein production. Furthermore, high glucose increased the mRNA expression levels of NLRP3, ACS, and caspase-1. Notably, NAC, a ROS scavenger, could attenuate high glucose-induced ROS formation and IL-18 and IL-1β mRNA and protein expression and block inflammasome activation. Silencing the NLRP3 gene expression also abolished IL-18 and IL-1β mRNA and protein expression. Intrudingly, H2S could ameliorate high glucose-induced ROS formation, IL-18 and IL-1β expression, and inflammasome activation. Taken together, the findings of the present study have demonstrated that H2S protects cultured RPE cells from high glucose-induced damage through inhibiting ROS formation and NLRP3 inflammasome activation. It might suggest that H2S represents a potential therapeutic target for the treatment of diabetic retinopathy.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

