L-Lactate Promotes Adult Hippocampal Neurogenesis
- PMID: 31178678
- PMCID: PMC6542996
- DOI: 10.3389/fnins.2019.00403
L-Lactate Promotes Adult Hippocampal Neurogenesis
Abstract
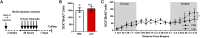
Neurogenesis, the formation of new neurons in the adult brain, is important for memory formation and extinction. One of the most studied external interventions that affect the rate of adult neurogenesis is physical exercise. Physical exercise promotes adult neurogenesis via several factors including brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF). Here, we identified L-lactate, a physical exercise-induced metabolite, as a factor that promotes adult hippocampal neurogenesis. While prolonged exposure to L-lactate promoted neurogenesis, no beneficial effect was exerted on cognitive learning and memory. Systemic pharmacological blocking of monocarboxylate transporter 2 (MCT2), which transports L-lactate to the brain, prevented lactate-induced neurogenesis, while 3,5-dihydroxybenzoic acid (3,5-DHBA), an agonist for the lactate-receptor hydroxycarboxylic acid receptor 1 (HCAR1), did not affect adult neurogenesis. These data suggest that L-lactate partially mediates the effect of physical exercise on adult neurogenesis, but not cognition, in a MCT2-dependent manner.
Keywords: L-lactate; MCT2; hippocampus; neurogenesis; neuronal progenitor cells.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

