The Effectiveness and Safety of Total Glucosides of Paeony in Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis
- PMID: 31178729
- PMCID: PMC6543198
- DOI: 10.3389/fphar.2019.00550
The Effectiveness and Safety of Total Glucosides of Paeony in Primary Sjögren's Syndrome: A Systematic Review and Meta-Analysis
Abstract
Objective: To assess the effectiveness and safety of the total glucosides of paeony (TGP) on the treatment of primary Sjögren's syndrome (pSS) by conducting a meta-analysis. Methods: Eight databases were searched from their inception to December 10, 2018 for randomized controlled trials (RCTs). The Revman 5.3 software was used for this meta-analysis. Results: Nine RCTs which included 770 participants were identified. Pooled results showed that significant difference in Schirmer's test (P < 0.00001) comparing TGP with placebo (PBO). However, the pooled results displayed significant differences in salivary flow rate, Schirmer's test, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), serum γ-globulin, immunoglobulin G (IgG), IgA, IgM, and effective rate (P ≤ 0.01) in the co-administration of TGP with immunosuppressant (IS) compared with IS alone. Subgroup analyses revealed both heterogeneities in ESR and serum γ-globulin were eliminated, showing combined intervention of TGP + IS being more advantageous than single usage of IS (P < 0.00001). However, the advantage varied among three subgroups and showed a gradual weakening over time. Furthermore, our results showed statistical significance in Schirmer's test (P = 0.0006), when hydroxychloroquine (HCQ) was jointly applied, but not in the case of combined TGP with methotrexate (MTX) (P = 0.41). For the safety analysis, the most common adverse events (AEs) were diarrhea or gastrointestinal discomfort, and no severe AEs were reported in TGP group. Meanwhile, six trials showed statistically insignificant differences between TGP + IS and IS in AEs (P = 0.76). Conclusions: Improving the lacrimal gland secretion (Schirmer's test) is the prominent function of TGP compared with PBO. TGP + IS can improve the clinical symptoms, such as lacrimal and salivary gland secretion function (Schirmer's test, salivary flow rate), inflammatory indices (ESR, CRP, and RF) and immunoglobulins (γ-globulin, IgG, IgA, and IgM) on the basis of IS monotherapy. In addition, TGP has an acceptable safety profile and AEs were not increased when TGP combined with IS in pSS. Therefore, TGP can be considered to be a potentially valid and safe drug for the treatment of pSS in the clinic. In view of the limitations of the included trials, the potential beneficial effectiveness and safety of TGP need additional high-quality, multi-center, and large-scale RCTs to assess its use in pSS treatment.
Keywords: effectiveness; meta-analysis; primary Sjögren's syndrome; safety; the total glucosides of paeony.
Figures
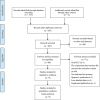







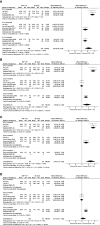

References
-
- Barone F., Colafrancesco S. (2016). Sjögren's syndrome: from pathogenesis to novel therapeutic targets. Clin. Exp. Rheumatol. 34, 58–62. - PubMed
-
- Cai W. H. (2011). Clinical investigation of Sjogren's syndrome treated with total glucosides of peony. Contem. Med. 17, 10–11. 10.3969/j.issn.1009-4393.2011.36.006 - DOI
-
- Carsons S. E., Vivino F. B., Parke A., Carteron A., Sankar V., Brasington R., et al. . (2017). Treatment guidelines for rheumatologic manifestations of sjogren's syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res. 69, 517–527. 10.1002/acr.22968 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

