Icariin Inhibits AGE-Induced Injury in PC12 Cells by Directly Targeting Apoptosis Regulator Bax
- PMID: 31178973
- PMCID: PMC6501163
- DOI: 10.1155/2019/7940808
Icariin Inhibits AGE-Induced Injury in PC12 Cells by Directly Targeting Apoptosis Regulator Bax
Abstract
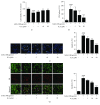
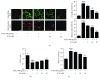
Diabetic encephalopathy (DE) is a serious complication caused by long-term cognitive impairment in diabetic patients. At present, there is no effective treatment for DE. Icariin (ICA) is a bioactive ingredient isolated from Epimedium. Previous research indicated that ICA was neuroprotective against Aβ-induced PC12 cell insult; however, the effect of ICA on an advanced glycosylation end product- (AGE-) induced neural injury model has not been studied. In this study, we investigated the neuroprotective effects of ICA on AGE-induced injury in PC12 cells. Our findings revealed that ICA could effectively protect PC12 cells from AGE-induced cell apoptosis by suppressing oxidative stress. Moreover, we observed that ICA could significantly protect against mitochondrial depolarization following AGE stimulation and inactivate the mitochondria-dependent caspase-9/3 apoptosis pathway. Most notably, we identified the direct target protein of ICA as apoptosis regulator Bax by a pulldown assay. We found that ICA could specifically target Bax protein and inhibit Bax dimer formation and migration to mitochondria. Furthermore, a siRNA knockdown experiment revealed that ICA could inhibit PC12 cell apoptosis and oxidative stress through targeting Bax. Taken together, our findings demonstrated that ICA could attenuate AGE-induced oxidative stress and mitochondrial apoptosis by specifically targeting Bax and further regulating the biological function of Bax on mitochondria.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

