Photosensitizer-Anchored 2D MOF Nanosheets as Highly Stable and Accessible Catalysts toward Artemisinin Production
- PMID: 31179208
- PMCID: PMC6548987
- DOI: 10.1002/advs.201802059
Photosensitizer-Anchored 2D MOF Nanosheets as Highly Stable and Accessible Catalysts toward Artemisinin Production
Abstract
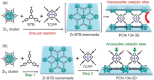
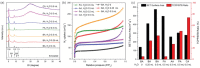
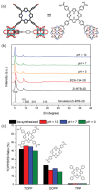
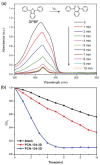
2D metal-organic frameworks (2D-MOFs) have recently emerged as promising materials for gas separations, sensing, conduction, and catalysis. However, the stability of these 2D-MOF catalysts and the tunability over catalytic environments are limited. Herein, it is demonstrated that 2D-MOFs can act as stable and highly accessible catalyst supports by introducing more firmly anchored photosensitizers as bridging ligands. An ultrathin MOF nanosheet-based material, Zr-BTB (BTB = 1,3,5-tris(4-carboxyphenyl)benzene), is initially constructed by connecting Zr6-clusters with the tritopic carboxylate linker. Surface modification of the Zr-BTB structure was realized through the attachment of porphyrin-based carboxylate ligands on the coordinatively unsaturated Zr metal sites in the MOF through strong Zr-carboxylate bond formation. The functionalized MOF nanosheet, namely PCN-134-2D, acts as an efficient photocatalyst for 1O2 generation and artemisinin production. Compared to the 3D analogue (PCN-134-3D), PCN-134-2D allows for fast reaction kinetics due to the enhanced accessibility of the catalytic sites within the structure and facile substrate diffusion. Additionally, PCN-134(Ni)-2D exhibits an exceptional yield of artemisinin, surpassing all reported homo- or heterogeneous photocatalysts for the artemisinin production.
Keywords: artemisinin; metal–organic frameworks; photochemical synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Novoselov K. S., Geim A. K., Morozov S. V., Jiang D., Zhang Y., Dubonos S. V., Grigorieva I. V., Firsov A. A., Science 2004, 306, 666. - PubMed
-
- Dean C. R., Young A. F., Meric I., Lee C., Wang L., Sorgenfrei S., Watanabe K., Taniguchi T., Kim P., Shepard K. L., Hone J., Nat. Nanotechnol. 2010, 5, 722. - PubMed
-
- Frindt R. F., J. Appl. Phys. 1966, 37, 1928.
-
- Naguib M., Kurtoglu M., Presser V., Lu J., Niu J., Heon M., Hultman L., Gogotsi Y., Barsoum M. W., Adv. Mater. 2011, 23, 4248. - PubMed
-
- Dubertret B., Heine T., Terrones M., Acc. Chem. Res. 2015, 48, 1. - PubMed
LinkOut - more resources
Full Text Sources
