MnmE, a Central tRNA-Modifying GTPase, Is Essential for the Growth, Pathogenicity, and Arginine Metabolism of Streptococcus suis Serotype 2
- PMID: 31179247
- PMCID: PMC6543552
- DOI: 10.3389/fcimb.2019.00173
MnmE, a Central tRNA-Modifying GTPase, Is Essential for the Growth, Pathogenicity, and Arginine Metabolism of Streptococcus suis Serotype 2
Abstract
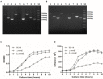
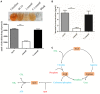
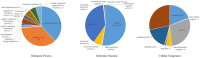
Streptococcus suis is an important pathogen in pigs and can also cause severe infections in humans. However, little is known about proteins associated with cell growth and pathogenicity of S. suis. In this study, a guanosine triphosphatase (GTPase) MnmE homolog was identified in a Chinese isolate (SC19) that drives a tRNA modification reaction. A mnmE deletion strain (ΔmnmE) and a complementation strain (CΔmnmE) were constructed to systematically decode the characteristics and functions of MnmE both in vitro and in vivo studies via proteomic analysis. Phenotypic analysis revealed that the ΔmnmE strain displayed deficient growth, attenuated pathogenicity, and perturbation of the arginine metabolic pathway mediated by the arginine deiminase system (ADS). Consistently, tandem mass tag -based quantitative proteomics analysis confirmed that 365 proteins were differentially expressed (174 up- and 191 down-regulated) between strains ΔmnmE and SC19. Many proteins associated with DNA replication, cell division, and virulence were down-regulated. Particularly, the core enzymes of the ADS were significantly down-regulated in strain ΔmnmE. These data also provide putative molecular mechanisms for MnmE in cell growth and survival in an acidic environment. Therefore, we propose that MnmE, by its function as a central tRNA-modifying GTPase, is essential for cell growth, pathogenicity, as well as arginine metabolism of S. suis.
Keywords: MnmE; Streptococcus suis (S. suis); arginine deiminase system; growth; pathogenicity; tRNA modifying guanosine triphosphatase; tandem mass tag-based quantitative proteomics.
Figures
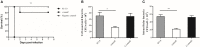

Similar articles
-
Proteomic and Metabolomic Analyses Provide Insights into the Mechanism on Arginine Metabolism Regulated by tRNA Modification Enzymes GidA and MnmE of Streptococcus suis.Front Cell Infect Microbiol. 2020 Dec 11;10:597408. doi: 10.3389/fcimb.2020.597408. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33425782 Free PMC article.
-
GidA, a tRNA Modification Enzyme, Contributes to the Growth, and Virulence of Streptococcus suis Serotype 2.Front Cell Infect Microbiol. 2016 Apr 19;6:44. doi: 10.3389/fcimb.2016.00044. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27148493 Free PMC article.
-
The Eukaryote-Like Serine/Threonine Kinase STK Regulates the Growth and Metabolism of Zoonotic Streptococcus suis.Front Cell Infect Microbiol. 2017 Mar 7;7:66. doi: 10.3389/fcimb.2017.00066. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28326294 Free PMC article.
-
tRNA modification enzymes GidA and MnmE: potential role in virulence of bacterial pathogens.Int J Mol Sci. 2014 Oct 10;15(10):18267-80. doi: 10.3390/ijms151018267. Int J Mol Sci. 2014. PMID: 25310651 Free PMC article. Review.
-
Invited review: MnmE, a GTPase that drives a complex tRNA modification reaction.Biopolymers. 2016 Aug;105(8):568-79. doi: 10.1002/bip.22813. Biopolymers. 2016. PMID: 26832457 Review.
Cited by
-
Deletion of lacD gene affected stress tolerance and virulence of Streptococcus suis serotype 2.J Microbiol. 2022 Sep;60(9):948-959. doi: 10.1007/s12275-022-2146-4. Epub 2022 Aug 19. J Microbiol. 2022. PMID: 35984615
-
tRNA modifying enzymes MnmE and MnmG are essential for Plasmodium falciparum apicoplast maintenance.bioRxiv [Preprint]. 2025 Jan 6:2024.12.21.629855. doi: 10.1101/2024.12.21.629855. bioRxiv. 2025. PMID: 39763917 Free PMC article. Preprint.
-
Peptidomics Analysis of Virulent Peptides Involved in Streptococcus suis Pathogenesis.Animals (Basel). 2021 Aug 24;11(9):2480. doi: 10.3390/ani11092480. Animals (Basel). 2021. PMID: 34573446 Free PMC article.
-
Evolutionary dynamics of the successful expansion of pandemic Vibrio parahaemolyticus ST3 in Latin America.Nat Commun. 2024 Sep 7;15(1):7828. doi: 10.1038/s41467-024-52159-y. Nat Commun. 2024. PMID: 39244587 Free PMC article.
-
One-carbon metabolism, folate, zinc and translation.Microb Biotechnol. 2020 Jul;13(4):899-925. doi: 10.1111/1751-7915.13550. Epub 2020 Mar 9. Microb Biotechnol. 2020. PMID: 32153134 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

