Anti-TNF Drugs for Chronic Uveitis in Adults-A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 31179280
- PMCID: PMC6543521
- DOI: 10.3389/fmed.2019.00104
Anti-TNF Drugs for Chronic Uveitis in Adults-A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
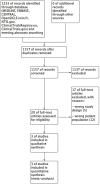
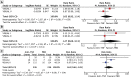
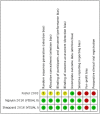
Background: We aimed to assess efficacy and safety of anti-tumor necrosis factor (TNF) drugs for adult chronic non-infectious uveitis (NIU). Methods: CENTRAL, MEDLINE, and EMBASE, were searched from inception to January 2019. Double-masked randomized placebo-controlled trials, assessing any anti-TNF vs. best medical intervention/standard of care in adults with chronic NIU were considered. The PRISMA and SAMPL guidelines were followed. The risk of bias was assessed using the Cochrane risk of bias tool. Overall quality of the evidence was assessed according to GRADE. PROSPERO registration: #CRD42016039068. The primary efficacy and safety outcomes were preservation of visual acuity (VA) and withdrawals due to adverse events, respectively. Meta-analysis of efficacy analysis was not performed due to significant clinical heterogeneity between studies' population and interventions. Results: A total of 1,157 references were considered and 3 studies were included. The overall risk of bias was moderate. In active NIU, adalimumab group showed an increased likelihood of VA preservation (risk ratio (RR) 1.75, 95%CI 1.32 to 2.32, n = 217), whereas the etanercept group did not (RR 0.81, 95%CI 0.57 to 1.14, n = 20). In inactive NIU, adalimumab was associated with increased likelihood of VA preservation (RR 1.31, 95%CI 1.12 to 1.53, n = 226). The rate of adverse events did not differ between anti-TNF and control arms (RR 1.03, 95%CI 0.94 to 1.13, n = 410). Conclusions: There is high quality evidence that adalimumab decreases the risk of worsening VA in active and inactive NIU and very low quality evidence that the risk of etanercept worsening VA in inactive NIU is not different from placebo. Moderate quality evidence suggests that anti-TNF agents are not different from placebo on the risk of study withdrawal.
Keywords: adalimumab; anti-tumor necrosis factor drugs; efficacy; etanercept; non-infectious uveitis; safety.
Figures
References
-
- Arcinue CA, Saboo US, Foster CS. Corticosteroids in uveitis. Nanomed. (2012) 2:55–64.
Publication types
LinkOut - more resources
Full Text Sources