Novel Hybrid Gels Made of High and Low Molecular Weight Hyaluronic Acid Induce Proliferation and Reduce Inflammation in an Osteoarthritis In Vitro Model Based on Human Synoviocytes and Chondrocytes
- PMID: 31179322
- PMCID: PMC6507116
- DOI: 10.1155/2019/4328219
Novel Hybrid Gels Made of High and Low Molecular Weight Hyaluronic Acid Induce Proliferation and Reduce Inflammation in an Osteoarthritis In Vitro Model Based on Human Synoviocytes and Chondrocytes
Erratum in
-
Corrigendum to "Novel Hybrid Gels Made of High and Low Molecular Weight Hyaluronic Acid Induce Proliferation and Reduce Inflammation in an Osteoarthritis In Vitro Model Based on Human Synoviocytes and Chondrocytes".Biomed Res Int. 2020 Jul 25;2020:7530149. doi: 10.1155/2020/7530149. eCollection 2020. Biomed Res Int. 2020. PMID: 32775439 Free PMC article.
Retraction in
-
Retracted: Novel Hybrid Gels Made of High and Low Molecular Weight Hyaluronic Acid Induce Proliferation and Reduce Inflammation in an Osteoarthritis In Vitro Model Based on Human Synoviocytes and Chondrocytes.Biomed Res Int. 2022 Feb 22;2022:9873639. doi: 10.1155/2022/9873639. eCollection 2022. Biomed Res Int. 2022. PMID: 35242877 Free PMC article.
Abstract
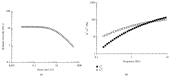
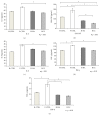
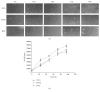
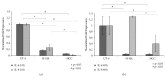
High molecular weight hyaluronan (H-HA) has a pivotal role in the maintenance of normal functions of synovial fluid and structure of the articular joint, but it has been shown that its concentration is reduced in patients affected by degenerative cartilage diseases, such as osteoarthritis (OA). The aim of this study was to investigate the anti-inflammatory effects and properties of hybrid cooperative complexes based on high and low molecular weight hyaluronan (HCC) compared to H-HA on human primary cells derived by pathological joints. In addition, the rheological behavior of HCC was evaluated in order to define their potential as viscosupplement gel in degenerated joints. The experiments were performed using an in vitro model of OA based on human chondrocytes and synoviocytes isolated from degenerated joints of patients hospitalized for surgical replacement. In order to assess the anti-inflammatory effects of HCC, we evaluated NF-kB, COMP-2, IL-6, and IL-8 as specific markers at the transcriptional and/or protein level. Moreover, the proliferative properties of HCC were assessed using time lapse video microscopy. We showed that chondrocytes and synoviocytes clearly presented an altered cytokine profile compatible with a severe ongoing inflammation status. H-HA and, above all, HCC significantly reduced levels of the specific biomarkers evaluated and improved cartilage healing. The rheological profile indicated HCC suitability for intra-articular injection in joint diseases. HCC viscoelastic properties and the protective/anti-inflammatory effect on human chondrocytes and synoviocytes suggest the novel HCC-based gels as a valid support for OA management.
Figures



References
-
- Hiligsmann M., Cooper C., Arden N., et al. Health economics in the field of osteoarthritis: an expert's consensus paper from the european society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO) Seminars in Arthritis and Rheumatism. 2013;43(3):303–313. doi: 10.1016/j.semarthrit.2013.07.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

