A Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus (CMV) in CMV-seropositive Recipients of Allogeneic Hematopoietic Cell Transplantation
- PMID: 31179485
- PMCID: PMC7146004
- DOI: 10.1093/cid/ciz490
A Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus (CMV) in CMV-seropositive Recipients of Allogeneic Hematopoietic Cell Transplantation
Abstract
Background: In a phase 3 trial, letermovir reduced clinically significant cytomegalovirus infections (CS-CMVi) and all-cause mortality at week 24 versus placebo in CMV-seropositive allogeneic hematopoietic cell transplantation (HCT) recipients. This post hoc analysis of phase 3 data further investigated the effects of letermovir on all-cause mortality.
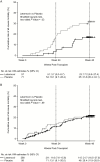
Methods: Kaplan-Meier survival curves were generated by treatment group for all-cause mortality. Observations were censored at trial discontinuation for reasons other than death or at trial completion. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using Cox modeling, adjusting for risk factors associated with mortality.
Results: Of 495 patients with no detectable CMV DNA at randomization, 437 had vital-status data available through week 48 post-HCT at trial completion (101 deaths, 20.4%). Following letermovir prophylaxis, the HR for all-cause mortality was 0.58 (95% CI, 0.35-0.98; P = .04) at week 24 and 0.74 (95% CI, 0.49-1.11; P = .14) at week 48 post-HCT versus placebo. Incidence of all-cause mortality through week 48 post-HCT in the letermovir group was similar in patients with or without CS-CMVi (15.8 vs 19.4%; P = .71). However, in the placebo group, all-cause mortality at week 48 post-HCT was higher in patients with versus those without CS-CMVi (31.0% vs 18.2%; P = .02). The HR for all-cause mortality in patients with CS-CMVi was 0.45 (95% CI, 0.21-1.00; P = .05) at week 48 for letermovir versus placebo.
Conclusions: Letermovir may reduce mortality by preventing or delaying CS-CMVi in HCT recipients.
Clinical trials registration: clinicaltrials.gov, NCT02137772.
Keywords: cytomegalovirus; hematopoietic cell transplantation; letermovir; mortality.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Cytomegalovirus Viremia and Death After Hematopoietic Cell Transplantation: More Complex Than "To Have and Have Not"?Clin Infect Dis. 2020 Apr 10;70(8):1534-1535. doi: 10.1093/cid/ciz492. Clin Infect Dis. 2020. PMID: 31179483 Free PMC article. No abstract available.
References
-
- Schmidt-Hieber M, Labopin M, Beelen D, et al. . CMV serostatus still has an important prognostic impact in de novo acute leukemia patients after allogeneic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood 2013; 122:3359–64. - PubMed
-
- Boeckh M, Leisenring W, Riddell SR, et al. . Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101:407–14. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical