Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children's Oncology Group
- PMID: 31180135
- PMCID: PMC6857800
- DOI: 10.1111/bjh.16014
Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children's Oncology Group
Abstract
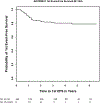
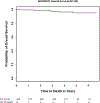
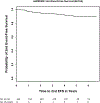
The AHOD0831 study for paediatric patients with high risk Hodgkin lymphoma tested a response-based approach designed to limit cumulative alkylator exposure and reduce radiation volumes. Patients (Stage IIIB/IVB) received two cycles of ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide). Rapid early responders [RER, no positron emission tomography (PET) activity above mediastinal blood pool] were consolidated with 2 cycles of ABVE-PC. Slow early responders (SER) received 2 cycles of ifosfamide/vinorelbine and 2 cycles of ABVE-PC. Radiotherapy was administered to sites of initial bulk and/or SER. By intent-to-treat analysis, 4-year second event-free survival (EFS; freedom from second relapse or malignancy) was 91·9% [95% confidence interval (CI): 86·1-95·3%], below the projected baseline of 95% (P = 0·038). Five-year first EFS and overall survival (OS) rates are 79·1% (95% CI: 71·5-84·8%) and 95% (95% CI: 88·8-97·8%). Eight of 11 SER patients with persistent PET positive lesions at the end of chemotherapy had clinical evidence of active disease (3 biopsy-proven, 5 with progressive disease or later relapses). Although this response-directed approach did not reach the ambitiously high pre-specified target for second EFS, EFS and OS rates are comparable with results of recent trials despite the reduction in radiotherapy volumes from historical involved fields. Persistent PET at end of chemotherapy identifies a cohort at an especially high risk for relapse/early progression.
Keywords: Hodgkin lymphoma; paediatric; positron emission tomography; radiation; response adapted.
© 2019 British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Conflicts of Interest:
The authors declare no competing financial interests.
Figures
Similar articles
-
Patterns of Initial Relapse from a Phase 3 Study of Response-Based Therapy for High-Risk Hodgkin Lymphoma (AHOD0831): A Report from the Children's Oncology Group.Int J Radiat Oncol Biol Phys. 2022 Mar 15;112(4):890-900. doi: 10.1016/j.ijrobp.2021.10.152. Epub 2021 Nov 9. Int J Radiat Oncol Biol Phys. 2022. PMID: 34767937 Free PMC article. Clinical Trial.
-
Patterns of relapse from a phase 3 Study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): a report from the Children's Oncology Group.Int J Radiat Oncol Biol Phys. 2015 May 1;92(1):60-6. doi: 10.1016/j.ijrobp.2014.10.042. Epub 2014 Dec 24. Int J Radiat Oncol Biol Phys. 2015. PMID: 25542311 Free PMC article. Clinical Trial.
-
Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children's Oncology Group Study AHOD0031.J Clin Oncol. 2014 Nov 10;32(32):3651-8. doi: 10.1200/JCO.2013.52.5410. Epub 2014 Oct 13. J Clin Oncol. 2014. PMID: 25311218 Free PMC article. Clinical Trial.
-
Limited-stage Hodgkin lymphoma: optimal chemotherapy and the role of radiotherapy.Am Soc Clin Oncol Educ Book. 2013:374-80. doi: 10.14694/EdBook_AM.2013.33.374. Am Soc Clin Oncol Educ Book. 2013. PMID: 23714551 Review.
-
European experience with ifosfamide in lymphomas.Semin Oncol. 1989 Feb;16(1 Suppl 3):73-7. Semin Oncol. 1989. PMID: 2468184 Review.
Cited by
-
Classical Hodgkin Lymphoma: From Past to Future-A Comprehensive Review of Pathophysiology and Therapeutic Advances.Int J Mol Sci. 2023 Jun 13;24(12):10095. doi: 10.3390/ijms241210095. Int J Mol Sci. 2023. PMID: 37373245 Free PMC article. Review.
-
Durable remission for four pediatric patients with high-risk relapsed classical Hodgkin lymphoma treated with brentuximab vedotin plus gemcitabine but without autologous stem cell transplantation: A report from the Children's Oncology Group.Pediatr Blood Cancer. 2022 Jun;69(6):e29649. doi: 10.1002/pbc.29649. Epub 2022 Mar 26. Pediatr Blood Cancer. 2022. PMID: 35338689 Free PMC article.
-
Prognostic value of baseline metabolic tumor volume in children and adolescents with intermediate-risk Hodgkin lymphoma treated with chemo-radiation therapy: FDG-PET parameter analysis in a subgroup from COG AHOD0031.Pediatr Blood Cancer. 2021 Sep;68(9):e29212. doi: 10.1002/pbc.29212. Epub 2021 Jul 10. Pediatr Blood Cancer. 2021. PMID: 34245210 Free PMC article.
-
Patterns of Initial Relapse from a Phase 3 Study of Response-Based Therapy for High-Risk Hodgkin Lymphoma (AHOD0831): A Report from the Children's Oncology Group.Int J Radiat Oncol Biol Phys. 2022 Mar 15;112(4):890-900. doi: 10.1016/j.ijrobp.2021.10.152. Epub 2021 Nov 9. Int J Radiat Oncol Biol Phys. 2022. PMID: 34767937 Free PMC article. Clinical Trial.
-
Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin's Lymphoma.N Engl J Med. 2022 Nov 3;387(18):1649-1660. doi: 10.1056/NEJMoa2206660. N Engl J Med. 2022. PMID: 36322844 Free PMC article. Clinical Trial.
References
-
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, Eich HT, Mueller-Hermelink HK, Kanz L, Greil R, Rank A, Paulus U, Smardova L, Huber C, Dorken B, Nerl C, Krause SW, Mueller RP, Fuchs M and Engert A (2011). “Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group.” J Clin Oncol 29(32): 4234–4242. - PubMed
-
- Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA, Zijlstra JM, Markova J, Meissner J, Feuring-Buske M, Huttmann A, Dierlamm J, Soekler M, Beck HJ, Willenbacher W, Ludwig WD, Pabst T, Topp MS, Hitz F, Bentz M, Keller UB, Kuhnhardt D, Ostermann H, Schmitz N, Hertenstein B, Aulitzky W, Maschmeyer G, Vieler T, Eich H, Baues C, Stein H, Fuchs M, Kuhnert G, Diehl V, Dietlein M and Engert A (2018). “PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group.” Lancet 390(10114): 2790–2802. - PubMed
-
- Casasnovas R-O, Bouabdallah R, Brice P, Lazarovici J, Ghesquieres H, Stamatoullas A, Dupuis J, Gac A-C, Gastinne T, Joly B, Bouabdallah K, Nicolas-Virelizier E, Feugier P, Morschhauser F, Delarue R, Farhat H, Quittet P, Berriolo-Riedinger A, Tempescul A, Edeline V, Maisonneuve H, Fornecker L-M, Lamy T, Delmer A, Dartigues P, Martin L, André M, Mounier N, Traverse-Glehen A and Meignan M (2019). “PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): a randomised, multicentre, non-inferiority, phase 3 study.” The Lancet Oncology 20(2): 202–215. - PubMed
-
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M & Diehl V (2007). “Revised response criteria for malignant lymphoma.” J Clin Oncol 25(5): 579–586. - PubMed
-
- Friedman DL, Chen L, Wolden S, Buxton A, McCarten K, FitzGerald TJ, Kessel S, De Alarcon PA, Chen AR, Kobrinsky N, Ehrlich P, Hutchison RE, Constine LS and Schwartz CL (2014). “Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031.” J Clin Oncol 32(32): 3651–3658. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

