Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS
- PMID: 31180318
- PMCID: PMC6557627
- DOI: 10.7554/eLife.45114
Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS
Abstract
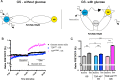
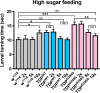
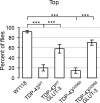
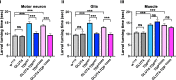
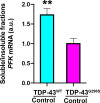
Amyotrophic Lateral Sclerosis (ALS), is a fatal neurodegenerative disorder, with TDP-43 inclusions as a major pathological hallmark. Using a Drosophila model of TDP-43 proteinopathy we found significant alterations in glucose metabolism including increased pyruvate, suggesting that modulating glycolysis may be neuroprotective. Indeed, a high sugar diet improves locomotor and lifespan defects caused by TDP-43 proteinopathy in motor neurons or glia, but not muscle, suggesting that metabolic dysregulation occurs in the nervous system. Overexpressing human glucose transporter GLUT-3 in motor neurons mitigates TDP-43 dependent defects in synaptic vesicle recycling and improves locomotion. Furthermore, PFK mRNA, a key indicator of glycolysis, is upregulated in flies and patient derived iPSC motor neurons with TDP-43 pathology. Surprisingly, PFK overexpression rescues TDP-43 induced locomotor deficits. These findings from multiple ALS models show that mechanistically, glycolysis is upregulated in degenerating motor neurons as a compensatory mechanism and suggest that increased glucose availability is protective.
Keywords: D. melanogaster; drosophila; human; iPSC; neuroscience.
© 2019, Manzo et al.
Conflict of interest statement
EM, IL, DB, AO, JB, AS, TK, BR, EL, DS, AJ, JL, RB, RS, DZ No competing interests declared
Figures











References
-
- Bakkar N, Kovalik T, Lorenzini I, Spangler S, Lacoste A, Sponaugle K, Ferrante P, Argentinis E, Sattler R, Bowser R. Artificial intelligence in neurodegenerative disease research: use of IBM Watson to identify additional RNA-binding proteins altered in amyotrophic lateral sclerosis. Acta Neuropathologica. 2018;135:227–247. doi: 10.1007/s00401-017-1785-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Gilliam Fellowship for Advanced Studies/HHMI/Howard Hughes Medical Institute/United States
- Undergraduate scholarship/Arnold and Mabel Beckman Foundation/International
- Post-Mortem Core/Target ALS/International
- R01 NS091299/NS/NINDS NIH HHS/United States
- Undergraduate Biology Research Program, Undergraduate scholarship/University of Arizona/International
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

