Changes in rapid HIV treatment initiation after national "treat all" policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis
- PMID: 31181056
- PMCID: PMC6557472
- DOI: 10.1371/journal.pmed.1002822
Changes in rapid HIV treatment initiation after national "treat all" policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis
Abstract
Background: Most countries have formally adopted the World Health Organization's 2015 recommendation of universal HIV treatment ("treat all"). However, there are few rigorous assessments of the real-world impact of treat all policies on antiretroviral treatment (ART) uptake across different contexts.
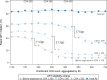
Methods and findings: We used longitudinal data for 814,603 patients enrolling in HIV care between 1 January 2004 and 10 July 2018 in 6 countries participating in the global International epidemiology Databases to Evaluate AIDS (IeDEA) consortium: Burundi (N = 11,176), Kenya (N = 179,941), Malawi (N = 84,558), Rwanda (N = 17,396), Uganda (N = 96,286), and Zambia (N = 425,246). Using a quasi-experimental regression discontinuity design, we assessed the change in the proportion initiating ART within 30 days of enrollment in HIV care (rapid ART initiation) after country-level adoption of the treat all policy. A modified Poisson model was used to identify factors associated with failure to initiate ART rapidly under treat all. In each of the 6 countries, over 60% of included patients were female, and median age at enrollment ranged from 32 to 36 years. In all countries studied, national adoption of treat all was associated with large increases in rapid ART initiation. Significant increases in rapid ART initiation immediately after treat all policy adoption were observed in Rwanda, from 44.4% to 78.9% of patients (34.5 percentage points [pp], 95% CI 27.2 to 41.7; p < 0.001), Kenya (25.7 pp, 95% CI 21.8 to 29.5; p < 0.001), Burundi (17.7 pp, 95% CI 6.5 to 28.9; p = 0.002), and Malawi (12.5 pp, 95% CI 7.5 to 17.5; p < 0.001), while no immediate increase was observed in Zambia (0.4 pp, 95% CI -2.9 to 3.8; p = 0.804) and Uganda (-4.2 pp, 95% CI -9.0 to 0.7; p = 0.090). The rate of rapid ART initiation accelerated sharply following treat all policy adoption in Malawi, Uganda, and Zambia; slowed in Kenya; and did not change in Rwanda and Burundi. In post hoc analyses restricted to patients enrolling under treat all, young adults (16-24 years) and men were at increased risk of not rapidly initiating ART (compared to older patients and women, respectively). However, rapid ART initiation following enrollment increased for all groups as more time elapsed since treat all policy adoption. Study limitations include incomplete data on potential ART eligibility criteria, such as clinical status, pregnancy, and enrollment CD4 count, which precluded the assessment of rapid ART initiation specifically among patients known to be eligible for ART before treat all.
Conclusions: Our analysis indicates that adoption of treat all policies had a strong effect on increasing rates of rapid ART initiation, and that these increases followed different trajectories across the 6 countries. Young adults and men still require additional attention to further improve rapid ART initiation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV Geneva: World Health Organization; 2015. - PubMed
-
- World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy Geneva: World Health Organization; 2017. - PubMed
-
- World Health Organization. WHO HIV policy adoption and implementation status in countries: fact sheet Geneva: World Health Organization; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

