AXL Is a Novel Predictive Factor and Therapeutic Target for Radioactive Iodine Refractory Thyroid Cancer
- PMID: 31181609
- PMCID: PMC6628138
- DOI: 10.3390/cancers11060785
AXL Is a Novel Predictive Factor and Therapeutic Target for Radioactive Iodine Refractory Thyroid Cancer
Abstract
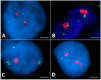
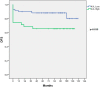
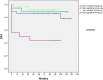
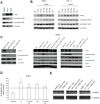
Papillary thyroid carcinomas (PTCs) have an excellent prognosis, but a fraction of them show aggressive behavior, becoming radioiodine (RAI)-resistant and/or metastatic. AXL (Anexelekto) is a tyrosine kinase receptor regulating viability, invasiveness and chemoresistance in various human cancers, including PTCs. Here, we analyze the role of AXL in PTC prognosis and as a marker of RAI refractoriness. Immunohistochemistry was used to assess AXL positivity in a cohort of human PTC samples. Normal and cancerous thyroid cell lines were used in vitro for signaling, survival and RAI uptake evaluations. 38.2% of human PTCs displayed high expression of AXL that positively correlated with RAI-refractoriness and disease persistence or recurrence, especially when combined with v-raf murine sarcoma viral oncogene homolog B(BRAF) V600E mutation. In human PTC samples, AXL expression correlated with V-akt murine thymoma viral oncogene homolog 1 (AKT1) and p65 nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) activation levels. Consistently, AXL stimulation with its ligand growth arrest-specific gene 6 (GAS6) increased AKT1- and p65 NF-kB-phosphorylation and promoted survival of thyroid cancer cell lines in culture. Enforced expression or activation of AXL in normal rat thyroid cells significantly reduced the expression of the sodium/iodide symporter (NIS) and the radioiodine uptake. These data indicate that AXL expression levels could be used as predictor of RAI refractoriness and as a possible novel therapeutic target of RAI resistant PTCs.
Keywords: AXL; disease persistence/recurrence; p65 NF-kB activation; radioactive iodine (RAI)-refractoriness; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The ability of anexelekto (AXL) expression and TERT promoter mutation to predict radioiodine-refractory differentiated thyroid carcinoma.Diagn Pathol. 2025 Apr 16;20(1):46. doi: 10.1186/s13000-025-01643-0. Diagn Pathol. 2025. PMID: 40241101 Free PMC article.
-
The molecular and gene/miRNA expression profiles of radioiodine resistant papillary thyroid cancer.J Exp Clin Cancer Res. 2020 Nov 16;39(1):245. doi: 10.1186/s13046-020-01757-x. J Exp Clin Cancer Res. 2020. PMID: 33198784 Free PMC article.
-
Radioiodine-Refractory Thyroid Cancer: Molecular Basis of Redifferentiation Therapies, Management, and Novel Therapies.Cancers (Basel). 2019 Sep 17;11(9):1382. doi: 10.3390/cancers11091382. Cancers (Basel). 2019. PMID: 31533238 Free PMC article. Review.
-
APOBEC SBS13 Mutational Signature-A Novel Predictor of Radioactive Iodine Refractory Papillary Thyroid Carcinoma.Cancers (Basel). 2022 Mar 21;14(6):1584. doi: 10.3390/cancers14061584. Cancers (Basel). 2022. PMID: 35326735 Free PMC article.
-
Radioactive Iodine-Refractory Differentiated Thyroid Cancer and Redifferentiation Therapy.Endocrinol Metab (Seoul). 2019 Sep;34(3):215-225. doi: 10.3803/EnM.2019.34.3.215. Endocrinol Metab (Seoul). 2019. PMID: 31565873 Free PMC article. Review.
Cited by
-
New insights into vitamin K biology with relevance to cancer.Trends Mol Med. 2022 Oct;28(10):864-881. doi: 10.1016/j.molmed.2022.07.002. Epub 2022 Aug 23. Trends Mol Med. 2022. PMID: 36028390 Free PMC article. Review.
-
PD-1 blockade delays tumor growth by inhibiting an intrinsic SHP2/Ras/MAPK signalling in thyroid cancer cells.J Exp Clin Cancer Res. 2021 Jan 7;40(1):22. doi: 10.1186/s13046-020-01818-1. J Exp Clin Cancer Res. 2021. PMID: 33413561 Free PMC article.
-
Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS.Theranostics. 2021 Apr 15;11(13):6251-6277. doi: 10.7150/thno.57689. eCollection 2021. Theranostics. 2021. PMID: 33995657 Free PMC article. Review.
-
Structure-based drug discovery of novel fused-pyrazolone carboxamide derivatives as potent and selective AXL inhibitors.Acta Pharm Sin B. 2023 Dec;13(12):4918-4933. doi: 10.1016/j.apsb.2023.10.002. Epub 2023 Oct 10. Acta Pharm Sin B. 2023. PMID: 38045061 Free PMC article.
-
Impact of Lin28 on lymph node metastasis in papillary thyroid carcinoma.Oncol Lett. 2021 Feb;21(2):97. doi: 10.3892/ol.2020.12358. Epub 2020 Dec 7. Oncol Lett. 2021. PMID: 33376530 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

