Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells
- PMID: 31181623
- PMCID: PMC6600362
- DOI: 10.3390/ijms20112792
Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells
Abstract
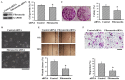
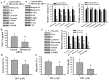
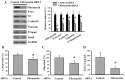
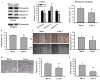
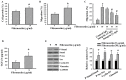
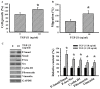
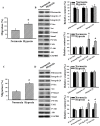
The prognostic and therapeutic values of fibronectin have been reported in patients with renal cell carcinoma (RCC). However, the underlying mechanisms of malignancy in RCC are not completely understood. We found that silencing of fibronectin expression attenuated human RCC 786-O and Caki-1 cell growth and migration. Silencing of potential fibronectin receptor integrin α5 and integrin β1 decreased 786-O cell ability in movement and chemotactic migration. Biochemical examination revealed a reduction of cyclin D1 and vimentin expression, transforming growth factor-β1 (TGF-β1) production, as well as Src and Smad phosphorylation in fibronectin-silenced 786-O and Caki-1 cells. Pharmacological inhibition of Src decreased 786-O cell growth and migration accompanied by a reduction of cyclin D1, fibronectin, vimentin, and TGF-β1 expression, as well as Src and Smad phosphorylation. In 786-O cells, higher activities in cell growth and migration than in Caki-1 cells were noted, along with elevated fibronectin and TGF-β1 expression. The additions of exogenous fibronectin and TGF-β1 promoted Caki-1 cell growth and migration, and increased cyclin D1, fibronectin, vimentin, and TGF-β1 expression, as well as Src and Smad phosphorylation. These findings highlight the role of fibronectin in RCC cell growth and migration involving Src and TGF-β1 signaling.
Keywords: extracellular matrix; fibronectin; motility; renal cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Fibronectin promotes nasopharyngeal cancer cell motility and proliferation.Biomed Pharmacother. 2019 Jan;109:1772-1784. doi: 10.1016/j.biopha.2018.11.055. Epub 2018 Nov 26. Biomed Pharmacother. 2019. PMID: 30551431
-
Runx2 silencing sensitized human renal cell carcinoma cells to ABT-737 apoptosis.Arch Biochem Biophys. 2024 Nov;761:110173. doi: 10.1016/j.abb.2024.110173. Epub 2024 Oct 5. Arch Biochem Biophys. 2024. PMID: 39369835
-
TGF-beta1-mediated activations of c-Src and Rac1 modulate levels of cyclins and p27(Kip1) CDK inhibitor in hepatoma cells replated on fibronectin.Biochim Biophys Acta. 2005 Mar 22;1743(1-2):151-61. doi: 10.1016/j.bbamcr.2004.09.014. Biochim Biophys Acta. 2005. PMID: 15777850
-
TGF-beta1 (transforming growth factor-beta1)-mediated adhesion of gastric carcinoma cells involves a decrease in Ras/ERKs (extracellular-signal-regulated kinases) cascade activity dependent on c-Src activity.Biochem J. 2004 Apr 1;379(Pt 1):141-50. doi: 10.1042/BJ20031408. Biochem J. 2004. PMID: 14720123 Free PMC article.
-
Cyclic stretch-induced TGF-β1 and fibronectin expression is mediated by β1-integrin through c-Src- and STAT3-dependent pathways in renal epithelial cells.Am J Physiol Renal Physiol. 2015 Mar 1;308(5):F425-36. doi: 10.1152/ajprenal.00589.2014. Epub 2014 Dec 4. Am J Physiol Renal Physiol. 2015. PMID: 25477471 Free PMC article.
Cited by
-
Application of Surface Plasmon Resonance Imaging Biosensors for Determination of Fibronectin, Laminin-5, and Type IV Collagen in Plasma, Urine, and Tissue of Renal Cell Carcinoma.Sensors (Basel). 2024 Sep 30;24(19):6371. doi: 10.3390/s24196371. Sensors (Basel). 2024. PMID: 39409411 Free PMC article.
-
The axis of tumor-associated macrophages, extracellular matrix proteins, and cancer-associated fibroblasts in oncogenesis.Cancer Cell Int. 2024 Oct 7;24(1):335. doi: 10.1186/s12935-024-03518-8. Cancer Cell Int. 2024. PMID: 39375726 Free PMC article. Review.
-
The Extracellular Matrix Environment of Clear Cell Renal Cell Carcinoma.Cancers (Basel). 2022 Aug 23;14(17):4072. doi: 10.3390/cancers14174072. Cancers (Basel). 2022. PMID: 36077607 Free PMC article. Review.
-
Impact of Extracellular Matrix Components to Renal Cell Carcinoma Behavior.Front Oncol. 2020 Apr 28;10:625. doi: 10.3389/fonc.2020.00625. eCollection 2020. Front Oncol. 2020. PMID: 32411604 Free PMC article.
-
Anticancer Activity of Rutin and Its Combination with Ionic Liquids on Renal Cells.Biomolecules. 2020 Feb 4;10(2):233. doi: 10.3390/biom10020233. Biomolecules. 2020. PMID: 32033222 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

