Sex Differences in the Epigenome: A Cause or Consequence of Sexual Differentiation of the Brain?
- PMID: 31181654
- PMCID: PMC6627918
- DOI: 10.3390/genes10060432
Sex Differences in the Epigenome: A Cause or Consequence of Sexual Differentiation of the Brain?
Abstract
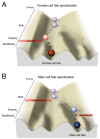
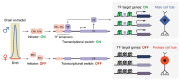
Females and males display differences in neural activity patterns, behavioral responses, and incidence of psychiatric and neurological diseases. Sex differences in the brain appear throughout the animal kingdom and are largely a consequence of the physiological requirements necessary for the distinct roles of the two sexes in reproduction. As with the rest of the body, gonadal steroid hormones act to specify and regulate many of these differences. It is thought that transient hormonal signaling during brain development gives rise to persistent sex differences in gene expression via an epigenetic mechanism, leading to divergent neurodevelopmental trajectories that may underlie sex differences in disease susceptibility. However, few genes with a persistent sex difference in expression have been identified, and only a handful of studies have employed genome-wide approaches to assess sex differences in epigenomic modifications. To date, there are no confirmed examples of gene regulatory elements that direct sex differences in gene expression in the brain. Here, we review foundational studies in this field, describe transcriptional mechanisms that could act downstream of hormone receptors in the brain, and suggest future approaches for identification and validation of sex-typical gene programs. We propose that sexual differentiation of the brain involves self-perpetuating transcriptional states that canalize sex-specific development.
Keywords: epigenetics; estrogen; gene regulation; neurodevelopment; sex differences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Gene regulatory mechanisms underlying sex differences in brain development and psychiatric disease.Ann N Y Acad Sci. 2018 May;1420(1):26-45. doi: 10.1111/nyas.13564. Epub 2018 Jan 24. Ann N Y Acad Sci. 2018. PMID: 29363776 Free PMC article. Review.
-
Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us.Horm Behav. 1996 Dec;30(4):627-61. doi: 10.1006/hbeh.1996.0066. Horm Behav. 1996. PMID: 9047287 Review.
-
Hormone-epigenome interactions in behavioural regulation.Horm Behav. 2020 Feb;118:104680. doi: 10.1016/j.yhbeh.2020.104680. Epub 2020 Jan 13. Horm Behav. 2020. PMID: 31927018 Review.
-
Epigenetic Mechanisms of Brain Sexual Differentiation.Cold Spring Harb Perspect Biol. 2022 Nov 1;14(11):a039099. doi: 10.1101/cshperspect.a039099. Cold Spring Harb Perspect Biol. 2022. PMID: 35817509 Free PMC article. Review.
-
Signatures of sex: Sex differences in gene expression in the vertebrate brain.Wiley Interdiscip Rev Dev Biol. 2020 Jan;9(1):e348. doi: 10.1002/wdev.348. Epub 2019 May 20. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31106965 Free PMC article. Review.
Cited by
-
Gender and sex in eating disorders: A narrative review of the current state of knowledge, research gaps, and recommendations.Brain Behav. 2023 Apr;13(4):e2871. doi: 10.1002/brb3.2871. Epub 2023 Feb 24. Brain Behav. 2023. PMID: 36840375 Free PMC article. Review.
-
Practical solutions for including sex as a biological variable (SABV) in preclinical neuropsychopharmacological research.J Neurosci Methods. 2024 Jan 1;401:110003. doi: 10.1016/j.jneumeth.2023.110003. Epub 2023 Oct 31. J Neurosci Methods. 2024. PMID: 37918446 Free PMC article. Review.
-
Flipping the parental switch: from killing to caring in male mammals.Anim Behav. 2020 Jul;165:133-142. doi: 10.1016/j.anbehav.2020.05.001. Epub 2020 Jun 25. Anim Behav. 2020. PMID: 37886700 Free PMC article.
-
A Meta-Analysis of Brain DNA Methylation Across Sex, Age, and Alzheimer's Disease Points for Accelerated Epigenetic Aging in Neurodegeneration.Front Aging Neurosci. 2021 Mar 11;13:639428. doi: 10.3389/fnagi.2021.639428. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33790779 Free PMC article.
-
Masculinized Second-to-Fourth Digit Ratio (2D:4D Ratio) Is Associated With Lower Cortisol Response in Infant Female Rhesus Monkeys (Macaca mulatta).Front Behav Neurosci. 2020 Sep 3;14:94. doi: 10.3389/fnbeh.2020.00094. eCollection 2020. Front Behav Neurosci. 2020. PMID: 33088262 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

