Investigating Prebiotic Protocells for A Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective
- PMID: 31181679
- PMCID: PMC6616946
- DOI: 10.3390/life9020049
Investigating Prebiotic Protocells for A Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective
Abstract
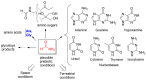
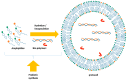
Protocells are supramolecular systems commonly used for numerous applications, such as the formation of self-evolvable systems, in systems chemistry and synthetic biology. Certain types of protocells imitate plausible prebiotic compartments, such as giant vesicles, that are formed with the hydration of thin films of amphiphiles. These constructs can be studied to address the emergence of life from a non-living chemical network. They are useful tools since they offer the possibility to understand the mechanisms underlying any living cellular system: Its formation, its metabolism, its replication and its evolution. Protocells allow the investigation of the synergies occurring in a web of chemical compounds. This cooperation can explain the transition between chemical (inanimate) and biological systems (living) due to the discoveries of emerging properties. The aim of this review is to provide an overview of relevant concept in prebiotic protocell research.
Keywords: compartment; origin of life; prebiotic chemistry; protocell; systems chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Framing major prebiotic transitions as stages of protocell development: three challenges for origins-of-life research.Beilstein J Org Chem. 2017 Jul 13;13:1388-1395. doi: 10.3762/bjoc.13.135. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28781704 Free PMC article.
-
The systems perspective at the crossroads between chemistry and biology.J Theor Biol. 2015 Sep 21;381:11-22. doi: 10.1016/j.jtbi.2015.04.036. Epub 2015 May 15. J Theor Biol. 2015. PMID: 25983045 Review.
-
From vesicles toward protocells and minimal cells.Soft Matter. 2022 Jul 6;18(26):4823-4849. doi: 10.1039/d1sm01695d. Soft Matter. 2022. PMID: 35722879 Review.
-
The origins of cellular life.Cold Spring Harb Perspect Biol. 2010 Sep;2(9):a002212. doi: 10.1101/cshperspect.a002212. Epub 2010 May 19. Cold Spring Harb Perspect Biol. 2010. PMID: 20484387 Free PMC article. Review.
-
Coacervate Microdroplets as Synthetic Protocells for Cell Mimicking and Signaling Communications.Small Methods. 2023 Dec;7(12):e2300042. doi: 10.1002/smtd.202300042. Epub 2023 Mar 12. Small Methods. 2023. PMID: 36908048 Review.
Cited by
-
Shape Deformation, Budding and Division of Giant Vesicles and Artificial Cells: A Review.Life (Basel). 2022 Jun 6;12(6):841. doi: 10.3390/life12060841. Life (Basel). 2022. PMID: 35743872 Free PMC article. Review.
-
Colocalization Analysis of Lipo-Deoxyribozyme Consisting of DNA and Protic Catalysts in a Vesicle-Based Protocellular Membrane Investigated by Confocal Microscopy.Life (Basel). 2021 Dec 8;11(12):1364. doi: 10.3390/life11121364. Life (Basel). 2021. PMID: 34947896 Free PMC article.
-
Prebiotic Organic Chemistry of Formamide and the Origin of Life in Planetary Conditions: What We Know and What Is the Future.Int J Mol Sci. 2021 Jan 18;22(2):917. doi: 10.3390/ijms22020917. Int J Mol Sci. 2021. PMID: 33477625 Free PMC article. Review.
-
Configurations of Proto-Cell Aggregates with Anisotropy: Gravity Promotes Complexity in Theoretical Biology.Entropy (Basel). 2022 Nov 3;24(11):1598. doi: 10.3390/e24111598. Entropy (Basel). 2022. PMID: 36359690 Free PMC article.
-
Protocells programmed through artificial reaction networks.Chem Sci. 2019 Dec 19;11(3):631-642. doi: 10.1039/c9sc05043d. Chem Sci. 2019. PMID: 34123035 Free PMC article. Review.
References
-
- Kitadai N., Maruyama S. Origins of building blocks of life: A review. Geosci. Front. 2018;9:1117–1153. doi: 10.1016/j.gsf.2017.07.007. - DOI
Publication types
LinkOut - more resources
Full Text Sources

