The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology
- PMID: 31181772
- PMCID: PMC6600236
- DOI: 10.3390/ijms20112810
The Evolving Role of CD8+CD28- Immunosenescent T Cells in Cancer Immunology
Abstract
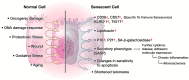
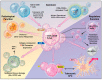
Functional, tumor-specific CD8+ cytotoxic T lymphocytes drive the adaptive immune response to cancer. Thus, induction of their activity is the ultimate aim of all immunotherapies. Success of anti-tumor immunotherapy is precluded by marked immunosuppression in the tumor microenvironment (TME) leading to CD8+ effector T cell dysfunction. Among the many facets of CD8+ T cell dysfunction that have been recognized-tolerance, anergy, exhaustion, and senescence-CD8+ T cell senescence is incompletely understood. Naïve CD8+ T cells require three essential signals for activation, differentiation, and survival through T-cell receptor, costimulatory receptors, and cytokine receptors. Downregulation of costimulatory molecule CD28 is a hallmark of senescent T cells and increased CD8+CD28- senescent populations with heterogeneous roles have been observed in multiple solid and hematogenous tumors. T cell senescence can be induced by several factors including aging, telomere damage, tumor-associated stress, and regulatory T (Treg) cells. Tumor-induced T cell senescence is yet another mechanism that enables tumor cell resistance to immunotherapy. In this paper, we provide a comprehensive overview of CD8+CD28- senescent T cell population, their origin, their function in immunology and pathologic conditions, including TME and their implication for immunotherapy. Further characterization and investigation into this subset of CD8+ T cells could improve the efficacy of future anti-tumor immunotherapy.
Keywords: CD8+CD28− T cells; cancer; cancer immunology; glioblastoma; immunotherapy; malignant glioma.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

