Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc
- PMID: 31181804
- PMCID: PMC6627379
- DOI: 10.3390/md17060341
Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc
Abstract
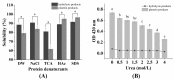
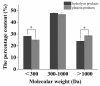
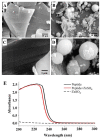
Zinc-binding peptides from oyster (Crassostrea gigas) have potential effects on zinc supplementation. The aim of this study was to prepare efficient zinc-binding peptides from oyster-modified hydrolysates by adding exogenous glutamate according to the plastein reaction and to further explore the zinc absorption mechanism of the peptide-zinc complex (MZ). The optimum conditions for the plastein reaction were as follows: pH 5.0, 40 °C, substrate concentration of 40%, pepsin dosage of 500 U/g, reaction time of 3 h and l-[1-13C]glutamate concentration of 10 mg/mL. The results of 13C isotope labelling suggested that the addition of l-[1-13C]glutamate contributed to the increase in the zinc-binding capacity of the peptide. The hydrophobic interaction was the main mechanism of action of the plastein reaction. Ultraviolet spectra and scanning electronic microscopy (SEM) revealed that the zinc-binding peptide could bind with zinc and form MZ. Furthermore, MZ could significantly enhance zinc bioavailability in the presence of phytic acid, compared to the commonly used ZnSO4. Additionally, MZ significantly promoted the intestinal absorption of zinc mainly through two pathways, the zinc ion channel and the small peptide transport pathway. Our work attempted to increase the understanding of the zinc absorption mechanism of MZ and to support the potential application of MZ as a supplementary medicine.
Keywords: caco-2 cells; intestinal absorption; oyster zinc-binding peptide; peptide-zinc complex; zinc bioavailability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Preparation and Characterization of an Oyster Peptide-Zinc Complex and Its Antiproliferative Activity on HepG2 Cells.Mar Drugs. 2023 Oct 18;21(10):542. doi: 10.3390/md21100542. Mar Drugs. 2023. PMID: 37888477 Free PMC article.
-
Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity.Food Chem. 2018 Aug 30;258:269-277. doi: 10.1016/j.foodchem.2018.03.030. Epub 2018 Mar 9. Food Chem. 2018. PMID: 29655733
-
Zinc in oysters (Crassostrea gigas): chemical characteristics and action during in vitro digestion.J Nutr Sci Vitaminol (Tokyo). 2003 Dec;49(6):405-8. doi: 10.3177/jnsv.49.405. J Nutr Sci Vitaminol (Tokyo). 2003. PMID: 14974730
-
Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans.Crit Rev Food Sci Nutr. 2023;63(19):3959-3979. doi: 10.1080/10408398.2021.1996328. Epub 2021 Oct 28. Crit Rev Food Sci Nutr. 2023. PMID: 34708681 Review.
-
Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides.Food Funct. 2016 Oct 12;7(10):4137-4144. doi: 10.1039/c6fo00706f. Food Funct. 2016. PMID: 27713952 Review.
Cited by
-
Intensive Value Utilization of Food-Derived Marine Immunoactive Peptides: Optimizing the Process Yield and Improving the Delivery Efficiency According to the Immune Activity Mechanism and Assisted Enzymatic Hydrolysis.Food Sci Nutr. 2025 Aug 5;13(8):e70578. doi: 10.1002/fsn3.70578. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40766787 Free PMC article. Review.
-
Novel Insights into Ethanol-Soluble Oyster Peptide-Zinc-Chelating Agents: Structural Characterization, Chelation Mechanism, and Potential Protection on MEHP-Induced Leydig Cells.Mar Drugs. 2024 Oct 10;22(10):465. doi: 10.3390/md22100465. Mar Drugs. 2024. PMID: 39452873 Free PMC article.
-
Development, characterization and in vivo zinc absorption capacity of a novel soy meal hydrolysate-zinc complexes.Front Nutr. 2023 Jul 6;10:1211609. doi: 10.3389/fnut.2023.1211609. eCollection 2023. Front Nutr. 2023. PMID: 37485380 Free PMC article.
-
Dietary Zn-Recent Advances in Studies on Its Bioaccessibility and Bioavailability.Molecules. 2025 Jun 25;30(13):2742. doi: 10.3390/molecules30132742. Molecules. 2025. PMID: 40649260 Free PMC article. Review.
-
Preparation and Characterization of an Oyster Peptide-Zinc Complex and Its Antiproliferative Activity on HepG2 Cells.Mar Drugs. 2023 Oct 18;21(10):542. doi: 10.3390/md21100542. Mar Drugs. 2023. PMID: 37888477 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

