Drug Delivery with Extracellular Vesicles: From Imagination to Innovation
- PMID: 31181910
- PMCID: PMC6639984
- DOI: 10.1021/acs.accounts.9b00109
Drug Delivery with Extracellular Vesicles: From Imagination to Innovation
Abstract
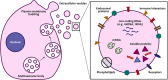
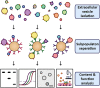
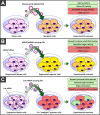
Extracellular vesicles are nanoparticles produced by cells. They are composed of cellular membrane with associated membrane proteins that surrounds an aqueous core containing soluble molecules such as proteins and nucleic acids, like miRNA and mRNA. They are important in many physiological and pathological processes as they can transfer biological molecules from producer cells to acceptor cells. Preparation of the niche for cancer metastasis, stimulation of tissue regeneration and orchestration of the immune response are examples of the diverse processes in which extracellular vesicles have been implicated. As a result, these vesicles have formed a source of inspiration for many scientific fields. They could be used, for example, as liquid biopsies in diagnostics, as therapeutics in regenerative medicine, or as drug delivery vehicles for transport of medicines. In this Account, we focus on drug delivery applications. As we learn more and more about these vesicles, the complexity increases. What originally appeared to be a relatively uniform population of cellular vesicles is increasingly subdivided into different subsets. Cells make various distinct vesicle types whose physicochemical aspects and composition is influenced by parental cell type, cellular activation state, local microenvironment, biogenesis pathway, and intracellular cargo sorting routes. It has proven difficult to assess the effects of changes in production protocol on the characteristics of the cell-derived vesicle population. On top of that, each isolation method for vesicles necessarily enriches certain vesicle classes and subpopulations while depleting others. Also, each method is associated with a varying degree of vesicle purity and concomitant coisolation of nonvesicular material. What emerges is a staggering heterogeneity. This constitutes one of the main challenges of the field as small changes in production and isolation protocols may have large impact on the vesicle characteristics and on subsequent vesicle activity. We try to meet this challenge by careful experimental design and development of tools that enable robust readouts. By engineering the surface and cargo of extracellular vesicles through chemical and biological techniques, favorable characteristics can be enforced while unfavorable qualities can be overruled or masked. This is coupled to the precise evaluation of the interaction of extracellular vesicles with cells to determine the extracellular vesicle uptake routes and intracellular routing. Sensitive reporter assays enable reproducible analysis of functional delivery. This systematic evaluation and optimization of extracellular vesicles improves our insight into the critical determinants of extracellular vesicle activity and should improve translation into clinical application of engineered extracellular vesicles as a new class of drug delivery systems.
Conflict of interest statement
The authors declare the following competing financial interest(s): R.M.S. is CSO of Excytex bv.
Figures
References
-
- Yanez-Mo M.; Siljander P. R.; Andreu Z.; Zavec A. B.; Borras F. E.; Buzas E. I.; Buzas K.; Casal E.; Cappello F.; Carvalho J.; Colas E.; Cordeiro-da Silva A.; Fais S.; Falcon-Perez J. M.; Ghobrial I. M.; Giebel B.; Gimona M.; Graner M.; Gursel I.; Gursel M.; Heegaard N. H.; Hendrix A.; Kierulf P.; Kokubun K.; Kosanovic M.; Kralj-Iglic V.; Kramer-Albers E. M.; Laitinen S.; Lasser C.; Lener T.; Ligeti E.; Line A.; Lipps G.; Llorente A.; Lotvall J.; Mancek-Keber M.; Marcilla A.; Mittelbrunn M.; Nazarenko I.; Nolte-’t Hoen E. N.; Nyman T. A.; O’Driscoll L.; Olivan M.; Oliveira C.; Pallinger E.; Del Portillo H. A.; Reventos J.; Rigau M.; Rohde E.; Sammar M.; Sanchez-Madrid F.; Santarem N.; Schallmoser K.; Ostenfeld M. S.; Stoorvogel W.; Stukelj R.; Van der Grein S. G.; Vasconcelos M. H.; Wauben M. H.; De Wever O. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066.10.3402/jev.v4.27066. - DOI - PMC - PubMed
-
- Vroman L.; Adams A. L.; Fischer G. C.; Munoz P. C. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood 1980, 55 (1), 156–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

