On trends and patterns in macroevolution: Williston's law and the branchiostegal series of extant and extinct osteichthyans
- PMID: 31182024
- PMCID: PMC6558815
- DOI: 10.1186/s12862-019-1436-x
On trends and patterns in macroevolution: Williston's law and the branchiostegal series of extant and extinct osteichthyans
Abstract
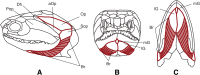
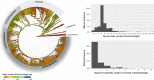
Background: The branchiostegal series consists of an alignment of bony elements in the posterior portion of the skull of osteichthyan vertebrates. We trace the evolution of the number of elements in a comprehensive survey that includes 440 extant and 66 extinct species. Using a newly updated actinopterygian tree in combination with phylogenetic comparative analyses, we test whether osteichthyan branchiostegals follow an evolutionary trend under 'Williston's law', which postulates that osteichthyan lineages experienced a reduction of bony elements over time.
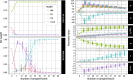
Results: We detected no overall macroevolutionary trend in branchiostegal numbers, providing no support for 'Williston's law'. This result is robust to the subsampling of palaeontological data, but the estimation of the model parameters is much more ambiguous.
Conclusions: We find substantial evidence for a macroevolutionary dynamic favouring an 'early burst' of trait evolution over alternative models. Our study highlights the challenges of accurately reconstructing macroevolutionary dynamics even with large amounts of data about extant and extinct taxa.
Keywords: Early burst; Evolutionary trend; Palaeontology; Phylogeny; Williston’s law.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Gregory WK, Roigneau M, Burr E, Evans G, Hellman E, Jackson F, et al. ‘Williston’s law’relating to the evolution of skull bones in the vertebrates. Am J Phys Anthropol. 1935;20:123–152. doi: 10.1002/ajpa.1330200202. - DOI
-
- Esteve-Altava B, Marugán-Lobón J, Botella H, Rasskin-Gutman D. Structural constraints in the evolution of the tetrapod skull complexity: Williston’s law revisited using network models. Evol Biol. 2013;40:209–219. doi: 10.1007/s11692-012-9200-9. - DOI
-
- Esteve-Altava B, Rasskin-Gutman D. Theoretical morphology of tetrapod skull networks. Comptes Rendus Palevol. 2014;13:41–50. doi: 10.1016/j.crpv.2013.08.003. - DOI
-
- McAllister DE. Evolution of branchiostegals and classification of teleostome fishes. Nat Mus Can Biol Ser. 1968;221:1–239.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

