Identifying genetic determinants of complex phenotypes from whole genome sequence data
- PMID: 31182025
- PMCID: PMC6558885
- DOI: 10.1186/s12864-019-5820-0
Identifying genetic determinants of complex phenotypes from whole genome sequence data
Abstract
Background: A critical goal in biology is to relate the phenotype to the genotype, that is, to find the genetic determinants of various traits. However, while simple monofactorial determinants are relatively easy to identify, the underpinnings of complex phenotypes are harder to predict. While traditional approaches rely on genome-wide association studies based on Single Nucleotide Polymorphism data, the ability of machine learning algorithms to find these determinants in whole proteome data is still not well known.
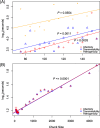
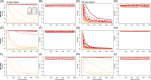
Results: To better understand the applicability of machine learning in this case, we implemented two such algorithms, adaptive boosting (AB) and repeated random forest (RRF), and developed a chunking layer that facilitates the analysis of whole proteome data. We first assessed the performance of these algorithms and tuned them on an influenza data set, for which the determinants of three complex phenotypes (infectivity, transmissibility, and pathogenicity) are known based on experimental evidence. This allowed us to show that chunking improves runtimes by an order of magnitude. Based on simulations, we showed that chunking also increases sensitivity of the predictions, reaching 100% with as few as 20 sequences in a small proteome as in the influenza case (5k sites), but may require at least 30 sequences to reach 90% on larger alignments (500k sites). While RRF has less specificity than random forest, it was never <50%, and RRF sensitivity was significantly higher at smaller chunk sizes. We then used these algorithms to predict the determinants of three types of drug resistance (to Ciprofloxacin, Ceftazidime, and Gentamicin) in a bacterium, Pseudomonas aeruginosa. While both algorithms performed well in the case of the influenza data, results were more nuanced in the bacterial case, with RRF making more sensible predictions, with smaller errors rates, than AB.
Conclusions: Altogether, we demonstrated that ML algorithms can be used to identify genetic determinants in small proteomes (viruses), even when trained on small numbers of individuals. We further showed that our RRF algorithm may deserve more scrutiny, which should be facilitated by the decreasing costs of both sequencing and phenotyping of large cohorts of individuals.
Keywords: Drug resistance; Genome-wide association study; Influenza virus; Machine learning; Pseudomonas aeruginosa.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

