Tenofovir Plasma Concentration from Preexposure Prophylaxis at the Time of Potential HIV Exposure: a Population Pharmacokinetic Modeling and Simulation Study Involving Serodiscordant Couples in East Africa
- PMID: 31182536
- PMCID: PMC6658796
- DOI: 10.1128/AAC.00446-19
Tenofovir Plasma Concentration from Preexposure Prophylaxis at the Time of Potential HIV Exposure: a Population Pharmacokinetic Modeling and Simulation Study Involving Serodiscordant Couples in East Africa
Abstract
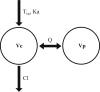
The Partners Demonstration Project was a prospective, open-label, implementation science-driven study of preexposure prophylaxis (PrEP) among heterosexual HIV serodiscordant couples in Kenya and Uganda. Adherence data were collected using the Medication Event Monitoring System (MEMS), and time of sexual activity was collected using the mobile phone short message service (SMS). Two plasma samples were collected at a single study visit. We integrated adherence, pharmacokinetics, and SMS data using a population pharmacokinetic (PopPK) model to simulate tenofovir plasma concentrations from PrEP at the time of sexual activity. In the first stage of this analysis, we used data from the current study to update a prior PopPK model of tenofovir (TFV) developed with data from the Partners PrEP Study (a phase III clinical trial). The second stage involved simulating plasma concentrations at the time of sexual activity using empirical Bayes estimates (EBEs) derived from the final model. In addition, EBEs from a previously published parent metabolite model of TFV (MTN-001, an open-label 3-way crossover study in healthy women) was used to simulate tenofovir diphosphate (TFV-DP) concentrations. We estimated percent PrEP "coverage" as the number of reported sexual events during which simulated concentrations were above an a priori threshold concentrations associated with a high degree of protection from HIV infection: plasma TFV of >40 ng/ml and peripheral blood mononuclear cell (PBMC) TFV-DP concentration of >36 fmol/million cells. The levels of coverage were 72% for TFV and 81% for TFV-DP. These levels are consistent with a high degree of protection against HIV acquisition in this study of a pragmatic delivery model for antiretroviral-based HIV prevention.
Keywords: PrEP; population pharmacokinetics; preexposure prophylaxis; tenofovir.
Copyright © 2019 American Society for Microbiology.
Figures




References
-
- US Department of Health and Human Services. 2012. FDA approves first drug for reducing the risk of sexually acquired HIV infection. https://aidsinfo.nih.gov/news/1254/fda-approves-first-drug-for-reducing-....
-
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363:2587–2599. doi:10.1056/NEJMoa1011205. - DOI - PMC - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. 2012. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 367:423–434. doi:10.1056/NEJMoa1110711. - DOI - PubMed
-
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. 2012. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 367:399–410. doi:10.1056/NEJMoa1108524. - DOI - PMC - PubMed
-
- Global Advocacy for HIV Prevention. 2017. Ongoing and planned PrEP demonstration and implementation studies. https://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and.... Accessed 7 May 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

