Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors
- PMID: 31182610
- PMCID: PMC6601006
- DOI: 10.1073/pnas.1903203116
Persistence of learning-induced synapses depends on neurotrophic priming of glucocorticoid receptors
Abstract
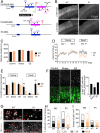
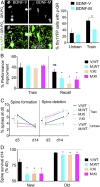
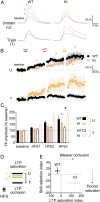
Stress can either promote or impair learning and memory. Such opposing effects depend on whether synapses persist or decay after learning. Maintenance of new synapses formed at the time of learning upon neuronal network activation depends on the stress hormone-activated glucocorticoid receptor (GR) and neurotrophic factor release. Whether and how concurrent GR and neurotrophin signaling integrate to modulate synaptic plasticity and learning is not fully understood. Here, we show that deletion of the neurotrophin brain-derived neurotrophic factor (BDNF)-dependent GR-phosphorylation (PO4) sites impairs long-term memory retention and maintenance of newly formed postsynaptic dendritic spines in the mouse cortex after motor skills training. Chronic stress and the BDNF polymorphism Val66Met disrupt the BDNF-dependent GR-PO4 pathway necessary for preserving training-induced spines and previously acquired memories. Conversely, enrichment living promotes spine formation but fails to salvage training-related spines in mice lacking BDNF-dependent GR-PO4 sites, suggesting it is essential for spine consolidation and memory retention. Mechanistically, spine maturation and persistence in the motor cortex depend on synaptic mobilization of the glutamate receptor subunit A1 (GluA1) mediated by GR-PO4 Together, these findings indicate that regulation of GR-PO4 via activity-dependent BDNF signaling is important for the formation and maintenance of learning-dependent synapses. They also define a signaling mechanism underlying these effects.
Keywords: BDNF-Val66Met; LTP; learning and memory; stress; two-photon microscopy.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance.Nat Neurosci. 2013 Jun;16(6):698-705. doi: 10.1038/nn.3387. Epub 2013 Apr 28. Nat Neurosci. 2013. PMID: 23624512 Free PMC article.
-
Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment.Proc Natl Acad Sci U S A. 2015 Dec 22;112(51):15737-42. doi: 10.1073/pnas.1509045112. Epub 2015 Nov 16. Proc Natl Acad Sci U S A. 2015. PMID: 26630005 Free PMC article.
-
Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation.Mol Cell Biol. 2013 Sep;33(18):3700-14. doi: 10.1128/MCB.00150-13. Epub 2013 Jul 22. Mol Cell Biol. 2013. PMID: 23878391 Free PMC article.
-
Linking Mitochondria to Synapses: New Insights for Stress-Related Neuropsychiatric Disorders.Neural Plast. 2016;2016:3985063. doi: 10.1155/2016/3985063. Epub 2016 Jan 14. Neural Plast. 2016. PMID: 26885402 Free PMC article. Review.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
Early life stress, literacy and dyslexia: an evolutionary perspective.Brain Struct Funct. 2024 May;229(4):809-822. doi: 10.1007/s00429-024-02766-8. Epub 2024 Mar 4. Brain Struct Funct. 2024. PMID: 38436668 Free PMC article. Review.
-
Dual imaging of dendritic spines and mitochondria in vivo reveals hotspots of plasticity and metabolic adaptation to stress.Neurobiol Stress. 2021 Sep 21;15:100402. doi: 10.1016/j.ynstr.2021.100402. eCollection 2021 Nov. Neurobiol Stress. 2021. PMID: 34611532 Free PMC article.
-
Meal scheduling corrects obesogenic diet induced-uncoupling of cortico-hippocampal activities supporting memory.EBioMedicine. 2025 Jul;117:105783. doi: 10.1016/j.ebiom.2025.105783. Epub 2025 Jun 16. EBioMedicine. 2025. PMID: 40527010 Free PMC article.
-
Disturbed Balance of Inhibitory Signaling Links Hearing Loss and Cognition.Front Neural Circuits. 2022 Jan 6;15:785603. doi: 10.3389/fncir.2021.785603. eCollection 2021. Front Neural Circuits. 2022. PMID: 35069123 Free PMC article.
-
Future Portrait of the Athletic Brain: Mechanistic Understanding of Human Sport Performance Via Animal Neurophysiology of Motor Behavior.Front Syst Neurosci. 2020 Nov 17;14:596200. doi: 10.3389/fnsys.2020.596200. eCollection 2020. Front Syst Neurosci. 2020. PMID: 33281568 Free PMC article.
References
-
- Lupien S. J., McEwen B. S., Gunnar M. R., Heim C., Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445 (2009). - PubMed
-
- Schwabe L., Joëls M., Roozendaal B., Wolf O. T., Oitzl M. S., Stress effects on memory: An update and integration. Neurosci. Biobehav. Rev. 36, 1740–1749 (2012). - PubMed
-
- Bramham C. R., Local protein synthesis, actin dynamics, and LTP consolidation. Curr. Opin. Neurobiol. 18, 524–531 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

