Single-site glycine-specific labeling of proteins
- PMID: 31182711
- PMCID: PMC6557831
- DOI: 10.1038/s41467-019-10503-7
Single-site glycine-specific labeling of proteins
Abstract
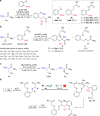
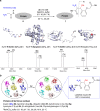
Labeling of native proteins invites interest from diverse segments of science. However, there remains the significant unmet challenge in precise labeling at a single site of a protein. Here, we report the site-specific labeling of natural or easy-to-engineer N-terminus Gly in proteins with remarkable efficiency and selectivity. The method generates a latent nucleophile from N-terminus imine that reacts with an aldehyde to deliver an aminoalcohol under physiological conditions. It differentiates N-Gly as a unique target amongst other proteinogenic amino acids. The method allows single-site labeling of proteins in isolated form and extends to lysed cells. It administers an orthogonal aldehyde group primed for late-stage tagging with an affinity tag, 19F NMR probe, and a fluorophore. A user-friendly protocol delivers analytically pure tagged proteins. The mild reaction conditions do not alter the structure and function of the protein. The cellular uptake of fluorophore-tagged insulin and its ability to activate the insulin-receptor mediated signaling remains unperturbed.
Conflict of interest statement
V.R. is the founder of Plabeltech Private Limited. A patent application has been filed on this work with V.R. and L.P. as inventors (Patent application no. WO-2018104962-A1, June 14, 2018). The remaining authors declare no competing interests.
Figures




Similar articles
-
Aldehydes can switch the chemoselectivity of electrophiles in protein labeling.Org Biomol Chem. 2018 Dec 12;16(48):9377-9381. doi: 10.1039/c8ob02897d. Org Biomol Chem. 2018. PMID: 30516786
-
Residue-specific N-terminal glycine to aldehyde transformation renders analytically pure single-site labeled proteins.Chem Commun (Camb). 2022 Nov 8;58(89):12451-12454. doi: 10.1039/d2cc04196k. Chem Commun (Camb). 2022. PMID: 36278269
-
Proximity-Induced Covalent Labeling of Proteins with a Reactive Fluorophore-Binding Peptide Tag.Bioconjug Chem. 2015 Aug 19;26(8):1466-9. doi: 10.1021/acs.bioconjchem.5b00304. Epub 2015 Jun 25. Bioconjug Chem. 2015. PMID: 26086394
-
Chemical biology-based approaches on fluorescent labeling of proteins in live cells.Mol Biosyst. 2013 May;9(5):862-72. doi: 10.1039/c2mb25422k. Mol Biosyst. 2013. PMID: 23318293 Review.
-
Reactivity and Selectivity Principles in Native Protein Bioconjugation.Chem Rec. 2021 Aug;21(8):1941-1956. doi: 10.1002/tcr.202100108. Epub 2021 Jun 28. Chem Rec. 2021. PMID: 34184826 Review.
Cited by
-
Site-Specific Labeling of Endogenous Proteins Using CoLDR Chemistry.J Am Chem Soc. 2021 Dec 8;143(48):20095-20108. doi: 10.1021/jacs.1c06167. Epub 2021 Nov 24. J Am Chem Soc. 2021. PMID: 34817989 Free PMC article.
-
Diversification of Phage-Displayed Peptide Libraries with Noncanonical Amino Acid Mutagenesis and Chemical Modification.Chem Rev. 2024 May 8;124(9):6051-6077. doi: 10.1021/acs.chemrev.4c00004. Epub 2024 Apr 30. Chem Rev. 2024. PMID: 38686960 Free PMC article. Review.
-
Selectivity and stability of N-terminal targeting protein modification chemistries.RSC Chem Biol. 2022 Nov 17;4(1):56-64. doi: 10.1039/d2cb00203e. eCollection 2023 Jan 4. RSC Chem Biol. 2022. PMID: 36685256 Free PMC article.
-
Targeting SUMOylation in Plasmodium as a Potential Target for Malaria Therapy.Front Cell Infect Microbiol. 2021 Jun 10;11:685866. doi: 10.3389/fcimb.2021.685866. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34178724 Free PMC article. Review.
-
Modification of N-terminal α-amine of proteins via biomimetic ortho-quinone-mediated oxidation.Nat Commun. 2021 Apr 15;12(1):2257. doi: 10.1038/s41467-021-22654-7. Nat Commun. 2021. PMID: 33859198 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

