Targeting CLDN18.2 by CD3 Bispecific and ADC Modalities for the Treatments of Gastric and Pancreatic Cancer
- PMID: 31182754
- PMCID: PMC6557842
- DOI: 10.1038/s41598-019-44874-0
Targeting CLDN18.2 by CD3 Bispecific and ADC Modalities for the Treatments of Gastric and Pancreatic Cancer
Erratum in
-
Author Correction: Targeting CLDN18.2 by CD3 Bispecific and ADC Modalities for the Treatments of Gastric and Pancreatic Cancer.Sci Rep. 2019 Nov 8;9(1):16735. doi: 10.1038/s41598-019-53130-4. Sci Rep. 2019. PMID: 31700121 Free PMC article.
Abstract
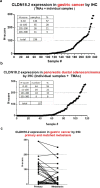
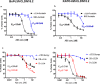
Human CLDN18.2 is highly expressed in a significant proportion of gastric and pancreatic adenocarcinomas, while normal tissue expression is limited to the epithelium of the stomach. The restricted expression makes it a potential drug target for the treatment of gastric and pancreatic adenocarcinoma, as evidenced by efforts to target CLDN18.2 via naked antibody and CAR-T modalities. Herein we describe CLDN18.2-targeting via a CD3-bispecific and an antibody drug conjugate and the characterization of these potential therapeutic molecules in efficacy and preliminary toxicity studies. Anti-hCLDN18.2 ADC, CD3-bispecific and diabody, targeting a protein sequence conserved in rat, mouse and monkey, exhibited in vitro cytotoxicity in BxPC3/hCLDN18.2 (IC50 = 1.52, 2.03, and 0.86 nM) and KATO-III/hCLDN18.2 (IC50 = 1.60, 0.71, and 0.07 nM) respectively and inhibited tumor growth of pancreatic and gastric patient-derived xenograft tumors. In a rat exploratory toxicity study, the ADC was tolerated up to 10 mg/kg. In a preliminary assessment of tolerability, the anti-CLDN18.2 diabody (0.34 mg/kg) did not produce obvious signs of toxicity in the stomach of NSG mice 4 weeks after dosing. Taken together, our data indicate that targeting CLDN18.2 with an ADC or bispecific modality could be a valid therapeutic approach for the treatment of gastric and pancreatic cancer.
Conflict of interest statement
All authors are current or former employees of Pfizer Inc.
Figures




References
-
- Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L., Torre Lindsey A., Jemal Ahmedin. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. - PubMed
-
- Pancreatic cancer statistics, https://www.wcrf.org/dietandcancer/cancer-trends/pancreatic-cancer-stati....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

