The Functions of DNA Damage Factor RNF8 in the Pathogenesis and Progression of Cancer
- PMID: 31182912
- PMCID: PMC6535783
- DOI: 10.7150/ijbs.31972
The Functions of DNA Damage Factor RNF8 in the Pathogenesis and Progression of Cancer
Abstract
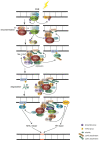
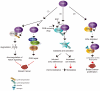
The really interesting new gene (RING) finger protein 8 (RNF8) is a central factor in DNA double strand break (DSB) signal transduction. DSB damage is the most toxic type of DNA damage to cells and is related to genomic instability. Multiple roles for RNF8 have been identified in DNA damage response as well as in other functions, such as telomere protection, cell cycle control and transcriptional regulation. These functions are closely correlated to tumorigenesis and cancer progression. Indeed, deficiency of RNF8 caused spontaneous tumorigenesis in a mouse model. Deciphering these mechanisms of RNF8 may shed light on strategies for cancer treatment. In this review, we summarize the current understanding of both classical and nonclassical functions of RNF8, and discuss its roles in the pathogenesis and progression of tumor.
Keywords: DSB; RNF8; cell cycle; telomere; transcriptional regulation; tumor.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Mitosis, double strand break repair, and telomeres: a view from the end: how telomeres and the DNA damage response cooperate during mitosis to maintain genome stability.Bioessays. 2014 Nov;36(11):1054-61. doi: 10.1002/bies.201400104. Epub 2014 Aug 29. Bioessays. 2014. PMID: 25171524 Free PMC article.
-
Rnf8 deficiency impairs class switch recombination, spermatogenesis, and genomic integrity and predisposes for cancer.J Exp Med. 2010 May 10;207(5):983-97. doi: 10.1084/jem.20092437. Epub 2010 Apr 12. J Exp Med. 2010. PMID: 20385750 Free PMC article.
-
Synergistic interaction of Rnf8 and p53 in the protection against genomic instability and tumorigenesis.PLoS Genet. 2013;9(1):e1003259. doi: 10.1371/journal.pgen.1003259. Epub 2013 Jan 31. PLoS Genet. 2013. PMID: 23382699 Free PMC article.
-
Opposing roles of RNF8/RNF168 and deubiquitinating enzymes in ubiquitination-dependent DNA double-strand break response signaling and DNA-repair pathway choice.J Radiat Res. 2016 Aug;57 Suppl 1(Suppl 1):i33-i40. doi: 10.1093/jrr/rrw027. Epub 2016 Mar 16. J Radiat Res. 2016. PMID: 26983989 Free PMC article. Review.
-
Fusing telomeres with RNF8.Nucleus. 2012 Mar 1;3(2):143-9. doi: 10.4161/nucl.19322. Epub 2012 Mar 1. Nucleus. 2012. PMID: 22555600 Free PMC article. Review.
Cited by
-
miR-27b-3p a Negative Regulator of DSB-DNA Repair.Genes (Basel). 2021 Aug 27;12(9):1333. doi: 10.3390/genes12091333. Genes (Basel). 2021. PMID: 34573315 Free PMC article.
-
Ubiquitin Ligases Involved in the Regulation of Wnt, TGF-β, and Notch Signaling Pathways and Their Roles in Mouse Development and Homeostasis.Genes (Basel). 2019 Oct 16;10(10):815. doi: 10.3390/genes10100815. Genes (Basel). 2019. PMID: 31623112 Free PMC article. Review.
-
RNF8 up-regulates AR/ARV7 action to contribute to advanced prostate cancer progression.Cell Death Dis. 2022 Apr 15;13(4):352. doi: 10.1038/s41419-022-04787-9. Cell Death Dis. 2022. PMID: 35428760 Free PMC article.
-
RNF8 depletion attenuates hepatocellular carcinoma progression by inhibiting epithelial-mesenchymal transition and enhancing drug sensitivity.Acta Biochim Biophys Sin (Shanghai). 2023 May 6;55(4):661-671. doi: 10.3724/abbs.2023076. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37154586 Free PMC article.
-
The Role of Ubiquitination in Osteosarcoma Development and Therapies.Biomolecules. 2024 Jul 3;14(7):791. doi: 10.3390/biom14070791. Biomolecules. 2024. PMID: 39062505 Free PMC article. Review.
References
-
- Seki N, Hattori A, Sugano S, Suzuki Y, Nakagawara A, Ohhira M. et al. Isolation, tissue expression, and chromosomal assignment of a novel human gene which encodes a protein with RING finger motif. J Hum Genet. 1998;43:272–4. - PubMed
-
- Ito K, Adachi S, Iwakami R, Yasuda H, Muto Y, Seki N. et al. N-Terminally extended human ubiquitin-conjugating enzymes (E2s) mediate the ubiquitination of RING-finger proteins, ARA54 and RNF8. Eur J Biochem. 2001;268:2725–32. - PubMed
-
- Plans V, Scheper J, Soler M, Loukili N, Okano Y, Thomson TM. The RING finger protein RNF8 recruits UBC13 for lysine 63-based self polyubiquitylation. J Cell Biochem. 2006;97:572–82. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

