Photocatalytic carbanion generation - benzylation of aliphatic aldehydes to secondary alcohols
- PMID: 31183069
- PMCID: PMC6524566
- DOI: 10.1039/c9sc01356c
Photocatalytic carbanion generation - benzylation of aliphatic aldehydes to secondary alcohols
Abstract
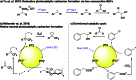
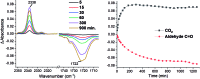
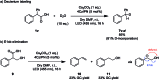
We present a redox-neutral method for the photocatalytic generation of carbanions. Benzylic carboxylates are photooxidized by single electron transfer; immediate CO2 extrusion and reduction of the in situ formed radical yields a carbanion capable of reacting with aliphatic aldehydes as electrophiles giving the Grignard analogous reaction product.
Figures


References
-
- Brahmachari G. RSC Adv. 2016;6:64676–64725.
-
- Ravelli D., Protti S., Fagnoni M. Chem. Rev. 2016;116:9850–9913. - PubMed
- Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C. Nat. Rev. Chem. 2017;1:0052.
- Prier C. K., Rankic D. A., MacMillan D. W. Chem. Rev. 2013;113:5322–5363. - PMC - PubMed
- Romero N. A., Nicewicz D. A. Chem. Rev. 2016;116:10075–10166. - PubMed
- Marzo L., Pagire S. K., Reiser O., König B. Angew. Chem., Int. Ed. 2018;57:10034–10072. - PubMed
- Ghosh I., Marzo L., Das A., Shaikh R., König B. Acc. Chem. Res. 2016;49:1566–1577. - PubMed
-
- Zhang Y., Qian R., Zheng X., Zeng Y., Sun J., Chen Y., Ding A., Guo H. Chem. Commun. 2015;51:54–57. - PubMed
- Liao L. L., Cao G. M., Ye J. H., Sun G. Q., Zhou W. J., Gui Y. Y., Yan S. S., Shen G., Yu D. G. J. Am. Chem. Soc. 2018;140:17338–17342. - PubMed
- Kumagai Y., Naoe T., Nishikawa K., Osaka K., Morita T., Yoshimi Y. Aust. J. Chem. 2015;68:1668.
- Yatham V. R., Shen Y., Martin R. Angew. Chem., Int. Ed. 2017;56:10915–10919. - PubMed
- Kong W., An H., Song Q. Chem. Commun. 2017;53:8968–8971. - PubMed
- Phelan J. P., Lang S. B., Compton J. S., Kelly C. B., Dykstra R., Gutierrez O., Molander G. A. J. Am. Chem. Soc. 2018;140:8037–8047. - PMC - PubMed
- Milligan J. A., Phelan J. P., Polites V. C., Kelly C. B., Molander G. A. Org. Lett. 2018;20:6840–6844. - PMC - PubMed
- Shu C., Mega R. S., Andreassen B. J., Noble A., Aggarwal V. K. Angew. Chem., Int. Ed. 2018;57:15430–15434. - PMC - PubMed
- Shu C., Noble A., Aggarwal V. K. Angew. Chem., Int. Ed. 2019;58:3870–3874. - PMC - PubMed
-
- Grignard V. C. R. Acad. Sci. 1900;130:1322–1324.
- Silverman G. S. and Rakita P. E., Handbook of Grignard Reagents, Marcel Dekker, Inc., New York, 1996.
LinkOut - more resources
Full Text Sources

