Magnetic Heating Stimulated Cargo Release with Dose Control using Multifunctional MR and Thermosensitive Liposome
- PMID: 31183312
- PMCID: PMC6536782
- DOI: 10.7150/ntno.31164
Magnetic Heating Stimulated Cargo Release with Dose Control using Multifunctional MR and Thermosensitive Liposome
Abstract
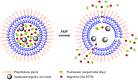
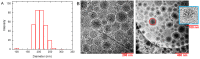
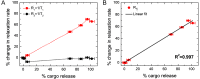
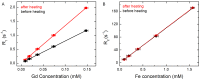
Rationale: Magnetic resonance imaging (MRI) is one of the most widely used diagnostic tools in the clinic. In this setting, real-time monitoring of therapy and tumor site would give the clinicians a handle to observe therapeutic response and to quantify drug amount to optimize the treatment. In this work, we developed a liposome-based cargo (cancer drugs) delivery strategy that could simultaneously monitor the real-time alternating magnetic field-induced cargo release from the change in MRI relaxation parameter R1 and the location and condition of liposome from the change in R2. The tumor site can then be monitored during the cargo release because liposomes would passively target the tumor site through the enhanced permeability and retention (EPR) effect. Physical insights from the experimental results and corresponding Monte Carlo spin dynamics simulations were also discussed. Methods: Superparamagnetic iron oxide (SPIO) nanoparticles, diethylenetriaminepentaacetic acid gadolinium(III) (Gd(III)-DTPA), and a model cancer drug (fluorescein) were co-loaded in PEGylated thermosensitive liposomes. The liposomes were characterized by transmission electron cryo-microscopy (cryoTEM), dynamic light scattering (DLS), and inductively coupled plasma optical emission spectrometry (ICP-OES). Alternating magnetic field (AMF) was used to create controlled mild hyperthermia (39-42°C) and facilitate controlled cargo (fluorescein) release from the thermosensitive liposomes. MRI relaxation parameters, R1 and R2, were measured at room temperature. The temporal variation in R1 was used to obtain the temporal profile of cargo release. Due to their similar sizes, both the gadolinium and cargo (model cancer drug fluorescein) would come out of the liposomes together as a result of heating. The temporal variation in R2 was used to monitor SPIO nanoparticles to enhance the tumor contrast. Monte Carlo spin dynamics simulations were performed by solving the Bloch equations and modeling SPIO nanoparticles as magnetized impenetrable spheres. Results: TEM images and DLS measurements showed the diameter of the liposome nanoparticle ~ 200 nm. AMF heating showed effective release of the model drug. It was found that R1 increased linearly by about 70% and then saturated as the cargo release process was completed, while R2 remained approximately constant with an initial 7%-drop and then recovered. The linear increase in R1 is consistent with the expected linear cargo release with time upon AMF heating. Monte Carlo spin dynamics simulations suggest that the initial temporal fluctuation of R2 is due to the plausible changes of SPIO aggregation and the slow non-recoverable degradation of liposomal membrane that increases water permeability with time by the heating process. The simulations show an order of magnitude increase in R2 at higher water permeability. Conclusion: We have performed MR parameter study of the release of a cargo (model cancer drug, fluorescein) by magnetic heating from thermosensitive multifunctional liposomes loaded with dual contrast agents. The size of the liposome nanoparticles loaded with model cancer drug (fluorescein), gadolinium chelate, and SPIO nanoparticles was appropriate for a variety of cancer therapies. A careful and detailed analysis with theoretical explanation and simulation was carried out to investigate the correlation between MRI relaxation parameters, R1 and R2, and different cargo release fractions. We have quantified the cargo release using R1, which shows a linear relation between each other. This result provides a strong basis for the dosage control of drug delivered. On the other hand, the fairly stable R2 with almost constant value suggests that it could be used to monitor the position and condition of the liposomal site, as SPIO nanoparticles mostly remained in the aqueous core of the liposome. Because our synthesized SPIO-encapsulated liposomes could be targeted to tumor site passively by the EPR effect, or actively through magnetofection, this study provides a solid ground for developing MR cancer theranostics in combination of this nanostructure and AMF heating strategy. Furthermore, our simulation results predict a sharp increase in R2 during the AMF heating, which opens up the exciting possibility of high-resolution, high-contrast real-time imaging of the liposomal site during the drug release process, provided AMF heating could be incorporated into an MRI setup. Our use of the clinically approved materials, along with confirmation by theoretical simulations, make this technique a promising candidate for translational MR cancer theranostics.
Keywords: AMF-controlled drug release; alternating magnetic field (AMF); magnetic hyperthermia; magnetic resonance theranostics; thermosensitive multifunctional liposome.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Rapid dynamic R1 /R2 */temperature assessment: a method with potential for monitoring drug delivery.NMR Biomed. 2014 Nov;27(11):1267-74. doi: 10.1002/nbm.3182. Epub 2014 Sep 10. NMR Biomed. 2014. PMID: 25208052
-
MRI contrast variation of thermosensitive magnetoliposomes triggered by focused ultrasound: a tool for image-guided local drug delivery.Contrast Media Mol Imaging. 2013 Mar-Apr;8(2):185-92. doi: 10.1002/cmmi.1515. Contrast Media Mol Imaging. 2013. PMID: 23281291
-
MRI monitoring of nanocarrier accumulation and release using Gadolinium-SPIO co-labelled thermosensitive liposomes.Contrast Media Mol Imaging. 2016 May;11(3):184-94. doi: 10.1002/cmmi.1679. Epub 2016 Jan 11. Contrast Media Mol Imaging. 2016. PMID: 26750715
-
Controlled Release of the Anticancer Drug Cyclophosphamide from a Superparamagnetic β-Cyclodextrin Nanosponge by Local Hyperthermia Generated by an Alternating Magnetic Field.ACS Appl Mater Interfaces. 2025 Mar 5;17(9):13001-13017. doi: 10.1021/acsami.3c18038. Epub 2024 Apr 19. ACS Appl Mater Interfaces. 2025. PMID: 38640460 Review.
-
Role of integrated cancer nanomedicine in overcoming drug resistance.Adv Drug Deliv Rev. 2013 Nov;65(13-14):1784-802. doi: 10.1016/j.addr.2013.07.012. Epub 2013 Jul 21. Adv Drug Deliv Rev. 2013. PMID: 23880506 Review.
Cited by
-
Doxorubicin-loaded liposomes surface engineered with the matrix metalloproteinase-2 cleavable polyethylene glycol conjugate for cancer therapy.Cancer Nanotechnol. 2023;14(1):18. doi: 10.1186/s12645-023-00169-8. Epub 2023 Mar 7. Cancer Nanotechnol. 2023. PMID: 36910721 Free PMC article.
-
Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery.Pharmaceutics. 2022 Mar 5;14(3):572. doi: 10.3390/pharmaceutics14030572. Pharmaceutics. 2022. PMID: 35335948 Free PMC article. Review.
-
Computational modeling of thermal combination therapies by magneto-ultrasonic heating to enhance drug delivery to solid tumors.Sci Rep. 2021 Oct 1;11(1):19539. doi: 10.1038/s41598-021-98554-z. Sci Rep. 2021. PMID: 34599207 Free PMC article.
-
Smart and Multi-Functional Magnetic Nanoparticles for Cancer Treatment Applications: Clinical Challenges and Future Prospects.Nanomaterials (Basel). 2022 Oct 12;12(20):3567. doi: 10.3390/nano12203567. Nanomaterials (Basel). 2022. PMID: 36296756 Free PMC article. Review.
-
Recent advances in nanoultrasonography for the diagnosis and treatment of gastrointestinal diseases.Nanomedicine (Lond). 2025 Mar;20(5):519-530. doi: 10.1080/17435889.2025.2457319. Epub 2025 Jan 23. Nanomedicine (Lond). 2025. PMID: 39846205 Review.
References
-
- Kelkar SS, Reineke TM. Theranostics: combining imaging and therapy. Bioconjug. Chem. 2011;22:1879–1903. - PubMed
-
- Sunderland CJ, Steiert M, Talmadge JE. et al. Targeted nanoparticles for detecting and treating cancer. Drug Dev. Res. 2006;67:70–93.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

