Pharmacokinetic and exposure-response analysis of pertuzumab in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer
- PMID: 31183514
- PMCID: PMC6682857
- DOI: 10.1007/s00280-019-03871-w
Pharmacokinetic and exposure-response analysis of pertuzumab in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer
Abstract
Purpose: To characterize the pharmacokinetics (PK) of pertuzumab and trastuzumab in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer in the randomized, double-blind, phase III JACOB study (NCT01774786), and to evaluate the appropriateness of the pertuzumab regimen in these patients.
Methods: Patients received 840 mg intravenous pertuzumab or placebo plus trastuzumab q3w and chemotherapy. Pertuzumab and trastuzumab were administered until disease progression or unacceptable toxicity. Chemotherapy was administered for up to six cycles or disease progression or unacceptable toxicity. Serum concentrations of pertuzumab and trastuzumab were measured. Pertuzumab PK was characterized across treatment cycles. The impact of anti-drug antibodies (ADAs) on pertuzumab PK and the impact of pertuzumab on trastuzumab PK were assessed. An exploratory exposure-efficacy analysis was also conducted.
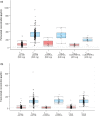
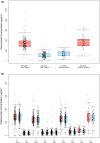
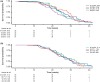
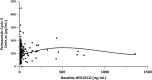
Results: In total, 374 patients in the pertuzumab arm had evaluable PK data. The mean observed pertuzumab steady-state serum trough (minimum) concentration (Cmin,ss) ± standard deviation was 114 ± 51.8 μg/mL. The target pertuzumab Cmin,ss of ≥ 20 μg/mL was reached in 99.3% of patients at Cycle 5 (steady state) and beyond. Greater than 90% of patients were above the PK target right after the first pertuzumab dose. There was no apparent impact of ADAs on pertuzumab PK nor of pertuzumab on trastuzumab PK. There were no differences in overall survival across Cycle 1 pertuzumab (Cmin) or Cycle 5 pertuzumab (Cmin,ss) exposure quartiles.
Conclusions: Pertuzumab exposure in JACOB was consistent with prior studies in advanced gastric cancer and breast cancer. The 840 mg q3w dose allowed the majority of patients in JACOB to achieve target pertuzumab concentrations and appears to be an appropriate dose selection.
Keywords: HER2-positive; Metastatic gastric cancer; Metastatic gastroesophageal junction; Pertuzumab; Pharmacokinetics; Trastuzumab.
Conflict of interest statement
All authors received support for third-party writing assistance for this manuscript, provided by F. Hoffmann-La Roche Ltd. Whitney Kirschbrown, Ihsan Nijem, Amit Garg, and Sandhya Girish are employees of Genentech, Inc., and hold stock in Roche Holding Ltd. Whitney Kirschbrown is an inventor on a pertuzumab-related filing. Bei Wang is an employee of Genentech, Inc. Atsushi Ohtsu has received research grants from BMS and honoraria from BMS, Ono, Chugai, and Taiho. Paulo M. Hoff has received research grants from Roche relating to the conduct of this study. Manish A. Shah discloses research funding paid to his institution from Boston Biomedical, Merck, and Roche. Lin Shen received non-financial support from Roche Pharmaceuticals Ltd. relating to the conduct of this study and a research grant from Hengrui Medicine Co. Ltd, indirectly related to this manuscript. Yoon-Koo Kang has received research grants from Roche, Daehwa and LSK Biopharma, and has received personal fees from Ono, BMS, Novartis, Lilly, Daehwa, and LSK Biopharma. Maria Alsina has performed an advisory role for BMS, Servier, and MSD, received research funding from Merck-Serono, and received speaker fees from MSD, BMS, Lilly, Roche, Amgen, and AstraZeneca.
Figures
Similar articles
-
Pertuzumab in combination with trastuzumab and chemotherapy for Chinese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subpopulation analysis of the JACOB trial.Cancer Commun (Lond). 2019 Jun 24;39(1):38. doi: 10.1186/s40880-019-0384-6. Cancer Commun (Lond). 2019. PMID: 31234927 Free PMC article. Clinical Trial.
-
Pertuzumab plus trastuzumab and chemotherapy for Japanese patients with HER2-positive metastatic gastric or gastroesophageal junction cancer: a subgroup analysis of the JACOB trial.Int J Clin Oncol. 2020 Feb;25(2):301-311. doi: 10.1007/s10147-019-01558-z. Epub 2019 Oct 16. Int J Clin Oncol. 2020. PMID: 31620931 Free PMC article. Clinical Trial.
-
Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study.Lancet Oncol. 2018 Oct;19(10):1372-1384. doi: 10.1016/S1470-2045(18)30481-9. Epub 2018 Sep 11. Lancet Oncol. 2018. PMID: 30217672 Clinical Trial.
-
Effect of pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer: A meta-analysis .Int J Clin Pharmacol Ther. 2017 Sep;55(9):720-727. doi: 10.5414/CP202921. Int J Clin Pharmacol Ther. 2017. PMID: 28737130
-
Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer.Drugs. 2013 Sep;73(13):1491-502. doi: 10.1007/s40265-013-0109-0. Drugs. 2013. PMID: 23982598 Review.
Cited by
-
Genomics and Targeted Therapies in Gastroesophageal Adenocarcinoma.Cancer Discov. 2019 Dec;9(12):1656-1672. doi: 10.1158/2159-8290.CD-19-0487. Epub 2019 Nov 14. Cancer Discov. 2019. PMID: 31727671 Free PMC article. Review.
-
A recombinant fragment antigen-binding (Fab) of trastuzumab displays low cytotoxic profile in adult human cardiomyocytes: first evidence and the key implication of FcγRIIA receptor.Acta Pharmacol Sin. 2025 Mar;46(3):618-631. doi: 10.1038/s41401-024-01397-3. Epub 2024 Oct 16. Acta Pharmacol Sin. 2025. PMID: 39414958
-
Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study.J Clin Oncol. 2021 Aug 20;39(24):2667-2675. doi: 10.1200/JCO.20.02822. Epub 2021 May 4. J Clin Oncol. 2021. PMID: 33945296 Free PMC article. Clinical Trial.
-
The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies.Front Immunol. 2020 Aug 18;11:1951. doi: 10.3389/fimmu.2020.01951. eCollection 2020. Front Immunol. 2020. PMID: 33013848 Free PMC article. Review.
-
Evaluation of Innate Immune System, Body Habitus, and Sex on the Pharmacokinetics and Pharmacodynamics of Anetumab Ravtansine in Patients With Cancer.Clin Transl Sci. 2025 Mar;18(3):e70178. doi: 10.1111/cts.70178. Clin Transl Sci. 2025. PMID: 40051118 Free PMC article. Clinical Trial.
References
-
- Genentech Inc (2018) Perjeta™ (pertuzumab). Prescribing Information (USA). https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125409s121lbl.pdf. Accessed 18 Dec 2018
-
- Roche Registration Ltd (2018) Perjeta® (pertuzumab). Summary of Product Characteristics. https://www.ema.europa.eu/documents/product-information/perjeta-epar-pro.... Accessed 18 Dec 2018
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

