Integration of purinergic and angiotensin II receptor function in renal vascular responses and renal injury in angiotensin II-dependent hypertension
- PMID: 31183668
- PMCID: PMC6635571
- DOI: 10.1007/s11302-019-09662-5
Integration of purinergic and angiotensin II receptor function in renal vascular responses and renal injury in angiotensin II-dependent hypertension
Abstract
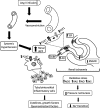
Glomerular arteriolar vasoconstriction and tubulointerstitial injury are observed before glomerular damage occurs in models of hypertension. High interstitial ATP concentrations, caused by the increase in arterial pressure, alter renal mechanisms involved in the long-term control of blood pressure, autoregulation of glomerular filtration rate and blood flow, tubuloglomerular feedback (TGF) responses, and sodium excretion. Elevated ATP concentrations and augmented expression of P2X receptors have been demonstrated under a genetic background or induction of hypertension with vasoconstrictor peptides. In addition to the alterations of the microcirculation in the hypertensive kidney, the vascular actions of elevated intrarenal angiotensin II levels may be mitigated by the administration of broad purinergic P2 antagonists or specific P2Y12, P2X1, and P2X7 receptor antagonists. Furthermore, the prevention of tubulointerstitial infiltration with immunosuppressor compounds reduces the development of salt-sensitive hypertension, indicating that tubulointerstitial inflammation is essential for the development and maintenance of hypertension. Inflammatory cells also express abundant purinergic receptors, and their activation by ATP induces cytokine and growth factor release that in turn contributes to augment tubulointerstitial inflammation. Collectively, the evidence suggests a pathophysiological activation of purinergic P2 receptors in angiotensin-dependent hypertension. Coexistent increases in intrarenal angiotensin II and activates Ang II AT1 receptors, which interacts with over-activated purinergic receptors in a complex manner, suggesting convergence of their post-receptor signaling processes.
Keywords: AT1 receptor antagonists; ATP; Angiotensin II; Hypertension; P2X antagonists; Purinergic P2X receptors; Renal hemodynamics.
Conflict of interest statement
Martha Franco declares that she has no conflict of interest.
Oscar Pérez-Mendez declares that he has no conflict of interest.
Supaporn Kulthinee declares that he has no conflict of interest.
L Gabriel Navar declares that he has no conflict of interest.
Figures


References
-
- Franco M, Martínez F, Rodríguez-Iturbe B, Johnson RJ, Santamaría J, Montoya A, Nepomuceno T, Bautista R, Tapia E, Herrera-Acosta J. Angiotensin II, interstitial inflammation and the pathogenesis of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2006;291:F1281–F1287. doi: 10.1152/ajprenal.00221.2006. - DOI - PubMed
-
- Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol. 2013;304:F982–F990. doi: 10.1152/ajprenal.00463.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

