Spontaneous Electrical Activity and Rhythmicity in Gastrointestinal Smooth Muscles
- PMID: 31183821
- PMCID: PMC7035145
- DOI: 10.1007/978-981-13-5895-1_1
Spontaneous Electrical Activity and Rhythmicity in Gastrointestinal Smooth Muscles
Abstract
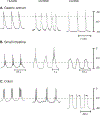
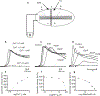
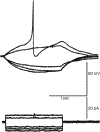
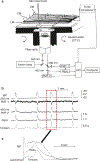
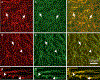
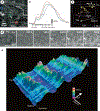
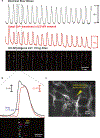
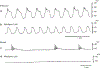
The gastrointestinal (GI) tract has multifold tasks of ingesting, processing, and assimilating nutrients and disposing of wastes at appropriate times. These tasks are facilitated by several stereotypical motor patterns that build upon the intrinsic rhythmicity of the smooth muscles that generate phasic contractions in many regions of the gut. Phasic contractions result from a cyclical depolarization/repolarization cycle, known as electrical slow waves, which result from intrinsic pacemaker activity. Interstitial cells of Cajal (ICC) are electrically coupled to smooth muscle cells (SMCs) and generate and propagate pacemaker activity and slow waves. The mechanism of slow waves is dependent upon specialized conductances expressed by pacemaker ICC. The primary conductances responsible for slow waves in mice are Ano1, Ca2+-activated Cl- channels (CaCCs), and CaV3.2, T-type, voltage-dependent Ca2+ channels. Release of Ca2+ from intracellular stores in ICC appears to be the initiator of pacemaker depolarizations, activation of T-type current provides voltage-dependent Ca2+ entry into ICC, as slow waves propagate through ICC networks, and Ca2+-induced Ca2+ release and activation of Ano1 in ICC amplifies slow wave depolarizations. Slow waves conduct to coupled SMCs, and depolarization elicited by these events enhances the open-probability of L-type voltage-dependent Ca2+ channels, promotes Ca2+ entry, and initiates contraction. Phasic contractions timed by the occurrence of slow waves provide the basis for motility patterns such as gastric peristalsis and segmentation. This chapter discusses the properties of ICC and proposed mechanism of electrical rhythmicity in GI muscles.
Keywords: ANO1 channels; Ca2+ transient; Electrophysiology; Gastrointestinal motility; Interstitial cells of Cajal; Pacemaker; SIP syncytium; Slow wave; T-type Ca2+ channels.
Figures
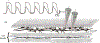




References
-
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

