Growth differentiation factor 11 locally controls anterior-posterior patterning of the axial skeleton
- PMID: 31183862
- PMCID: PMC6772169
- DOI: 10.1002/jcp.28904
Growth differentiation factor 11 locally controls anterior-posterior patterning of the axial skeleton
Abstract
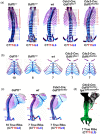
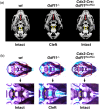
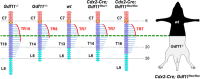
Growth and differentiation factor 11 (GDF11) is a transforming growth factor β family member that has been identified as the central player of anterior-posterior (A-P) axial skeletal patterning. Mice homozygous for Gdf11 deletion exhibit severe anterior homeotic transformations of the vertebrae and craniofacial defects. During early embryogenesis, Gdf11 is expressed predominantly in the primitive streak and tail bud regions, where new mesodermal cells arise. On the basis of this expression pattern of Gdf11 and the phenotype of Gdf11 mutant mice, it has been suggested that GDF11 acts to specify positional identity along the A-P axis either by local changes in levels of signaling as development proceeds or by acting as a morphogen. To further investigate the mechanism of action of GDF11 in the vertebral specification, we used a Cdx2-Cre transgene to generate mosaic mice in which Gdf11 expression is removed in posterior regions including the tail bud, but not in anterior regions. The skeletal analysis revealed that these mosaic mice display patterning defects limited to posterior regions where Gdf11 expression is deficient, whereas displaying normal skeletal phenotype in anterior regions where Gdf11 is normally expressed. Specifically, the mosaic mice exhibited seven true ribs, a pattern observed in wild-type (wt) mice (vs. 10 true ribs in Gdf11-/- mice), in the anterior axis and nine lumbar vertebrae, a pattern observed in Gdf11 null mice (vs. six lumbar vertebrae in wt mice), in the posterior axis. Our findings suggest that GDF11, rather than globally acting as a morphogen secreted from the tail bud, locally regulates axial vertebral patterning.
Keywords: Cdx2-Cre; GDF11; skeletal patterning; tail bud.
© 2019 The Authors Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Similar sequences but dissimilar biological functions of GDF11 and myostatin.Exp Mol Med. 2020 Oct;52(10):1673-1693. doi: 10.1038/s12276-020-00516-4. Epub 2020 Oct 19. Exp Mol Med. 2020. PMID: 33077875 Free PMC article. Review.
-
Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11.Nat Genet. 1999 Jul;22(3):260-4. doi: 10.1038/10320. Nat Genet. 1999. PMID: 10391213
-
Growth differentiation factor 11 signaling controls retinoic acid activity for axial vertebral development.Dev Biol. 2010 Nov 1;347(1):195-203. doi: 10.1016/j.ydbio.2010.08.022. Epub 2010 Aug 27. Dev Biol. 2010. PMID: 20801112 Free PMC article.
-
Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb.Dev Biol. 2001 Jan 15;229(2):407-20. doi: 10.1006/dbio.2000.9981. Dev Biol. 2001. PMID: 11203700
-
Axis development: the mouse becomes a dachshund.Curr Biol. 1999 Oct 21;9(20):R783-6. doi: 10.1016/S0960-9822(00)80013-5. Curr Biol. 1999. PMID: 10531023 Review.
Cited by
-
Protogenin facilitates trunk-to-tail HOX code transition via modulating GDF11/SMAD2 signaling in mammalian embryos.Commun Biol. 2024 Dec 19;7(1):1669. doi: 10.1038/s42003-024-07342-8. Commun Biol. 2024. PMID: 39702818 Free PMC article.
-
Gdf11 regulates left-right asymmetry development through TGF-β signal.Cell Prolif. 2025 Mar;58(3):e13765. doi: 10.1111/cpr.13765. Epub 2024 Oct 15. Cell Prolif. 2025. PMID: 39407407 Free PMC article.
-
The WNT7A/WNT7B/GPR124/RECK signaling module plays an essential role in mammalian limb development.Development. 2022 May 1;149(9):dev200340. doi: 10.1242/dev.200340. Epub 2022 May 12. Development. 2022. PMID: 35552394 Free PMC article.
-
Similar sequences but dissimilar biological functions of GDF11 and myostatin.Exp Mol Med. 2020 Oct;52(10):1673-1693. doi: 10.1038/s12276-020-00516-4. Epub 2020 Oct 19. Exp Mol Med. 2020. PMID: 33077875 Free PMC article. Review.
-
hPSC-derived sacral neural crest enables rescue in a severe model of Hirschsprung's disease.Cell Stem Cell. 2023 Mar 2;30(3):264-282.e9. doi: 10.1016/j.stem.2023.02.003. Cell Stem Cell. 2023. PMID: 36868194 Free PMC article.
References
-
- Agarwal, A. , Wu, P. H. , Hughes, E. G. , Fukaya, M. , Tischfield, M. A. , Langseth, A. J. , … Bergles, D. E. (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron, 93(3), 587–605. 10.1016/j.neuron.2016.12.034e587 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

