Identification of a Novel Allosteric Modulator of the Human Dopamine Transporter
- PMID: 31184115
- PMCID: PMC6703927
- DOI: 10.1021/acschemneuro.9b00262
Identification of a Novel Allosteric Modulator of the Human Dopamine Transporter
Abstract
The dopamine transporter (DAT) serves a pivotal role in controlling dopamine (DA)-mediated neurotransmission by clearing DA from synaptic and perisynaptic spaces and controlling its action at postsynaptic DA receptors. Major drugs of abuse such as amphetamine and cocaine interact with DAT to mediate their effects by enhancing extracellular DA concentrations. We previously identified a novel allosteric site in the related human serotonin transporter that lies outside the central substrate and inhibitor binding pocket. We used the hybrid structure based (HSB) method to screen for allosteric modulator molecules that target a similar site in DAT. We identified a compound, KM822, that was found to be a selective, noncompetitive inhibitor of DAT. We confirmed the structural determinants of KM822 allosteric binding within the allosteric site by structure/function and substituted cysteine scanning accessibility biotinylation experiments. In the in vitro cell-based assay and ex vivo in both rat striatal synaptosomal and slice preparations, KM822 was found to decrease the affinity of cocaine for DAT. The in vivo effects of KM822 on cocaine were tested on psychostimulant-associated behaviors in a planarian model where KM822 specifically inhibited the locomotion elicited by DAT-interacting stimulants amphetamine and cocaine. Overall, KM822 provides a unique opportunity as a molecular probe to examine allosteric modulation of DAT function.
Keywords: Allosteric modulation; cocaine use disorder; dopamine transporter; hybrid structure based method; substituted cysteine scanning accessibility method.
Conflict of interest statement
The authors declare the following competing financial interest(s): KM822 and its analogues are listed in the US Patent 9616065 with S.K., O.V.M., A.C.K.F., and J.M.S. as named inventors.
Figures




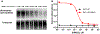




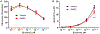

References
-
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, and Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 63, 585–640. - PubMed
-
- Yamashita A, Singh SK, Kawate T, Jin Y, and Gouaux E (2005) Crystal structure of a bacterial homologue of Na+/Cl– dependent neurotransmitter transporters. Nature 437, 215–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

