PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway
- PMID: 31184561
- PMCID: PMC6677282
- DOI: 10.1148/radiol.2019182946
PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway
Abstract
High-quality evidence shows that MRI in biopsy-naive men can reduce the number of men who need prostate biopsy and can reduce the number of diagnoses of clinically insignificant cancers that are unlikely to cause harm. In men with prior negative biopsy results who remain under persistent suspicion, MRI improves the detection and localization of life-threatening prostate cancer with greater clinical utility than the current standard of care, systematic transrectal US-guided biopsy. Systematic analyses show that MRI-directed biopsy increases the effectiveness of the prostate cancer diagnosis pathway. The incorporation of MRI-directed pathways into clinical care guidelines in prostate cancer detection has begun. The widespread adoption of the Prostate Imaging Reporting and Data System (PI-RADS) for multiparametric MRI data acquisition, interpretation, and reporting has promoted these changes in practice. The PI-RADS MRI-directed biopsy pathway enables the delivery of key diagnostic benefits to men suspected of having cancer based on clinical suspicion. Herein, the PI-RADS Steering Committee discusses how the MRI pathway should be incorporated into routine clinical practice and the challenges in delivering the positive health impacts needed by men suspected of having clinically significant prostate cancer.
© RSNA, 2019.
Figures

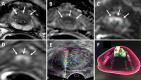
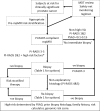
References
-
- Turkbey B, Rosenkrantz AB, Haider MA, et al. . Prostate Imaging Reporting and Data System version 2.1: 2019 update of Prostate Imaging Reporting and Data System version 2. Eur Urol 2019 Mar 18 [Epub ahead of print]. - PubMed
-
- Stabile A, Giganti F, Emberton M, Moore CM. MRI in prostate cancer diagnosis: do we need to add standard sampling? a review of the last 5 years. Prostate Cancer Prostatic Dis 2018;21(4):473–487. - PubMed
-
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. . Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389(10071):815–822. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

