Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke
- PMID: 31184741
- PMCID: PMC6563564
- DOI: 10.1001/jama.2019.7108
Association Between Transcatheter Aortic Valve Replacement for Bicuspid vs Tricuspid Aortic Stenosis and Mortality or Stroke
Abstract
Importance: Transcatheter aortic valve replacement (TAVR) indications are expanding, leading to an increasing number of patients with bicuspid aortic stenosis undergoing TAVR. Pivotal randomized trials conducted to obtain US Food and Drug Administration approval excluded bicuspid anatomy.
Objective: To compare the outcomes of TAVR with a balloon-expandable valve for bicuspid vs tricuspid aortic stenosis.
Design, setting, and participants: Registry-based prospective cohort study of patients undergoing TAVR at 552 US centers. Participants were enrolled in the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapies Registry from June 2015 to November 2018.
Exposures: TAVR for bicuspid vs tricuspid aortic stenosis.
Main outcomes and measures: Primary outcomes were 30-day and 1-year mortality and stroke. Secondary outcomes included procedural complications, valve hemodynamics, and quality of life assessment.
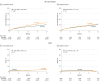
Results: Of 81 822 consecutive patients with aortic stenosis (2726 bicuspid; 79 096 tricuspid), 2691 propensity-score matched pairs of bicuspid and tricuspid aortic stenosis were analyzed (median age, 74 years [interquartile range {IQR}, 66-81 years]; 39.1%, women; mean [SD] STS-predicted risk of mortality, 4.9% [4.0%] and 5.1% [4.2%], respectively). All-cause mortality was not significantly different between patients with bicuspid and tricuspid aortic stenosis at 30 days (2.6% vs 2.5%; hazard ratio [HR], 1.04, [95% CI, 0.74-1.47]) and 1 year (10.5% vs 12.0%; HR, 0.90 [95% CI, 0.73-1.10]). The 30-day stroke rate was significantly higher for bicuspid vs tricuspid aortic stenosis (2.5% vs 1.6%; HR, 1.57 [95% CI, 1.06-2.33]). The risk of procedural complications requiring open heart surgery was significantly higher in the bicuspid vs tricuspid cohort (0.9% vs 0.4%, respectively; absolute risk difference [RD], 0.5% [95% CI, 0%-0.9%]). There were no significant differences in valve hemodynamics. There were no significant differences in moderate or severe paravalvular leak at 30 days (2.0% vs 2.4%; absolute RD, 0.3% [95% CI, -1.3% to 0.7%]) and 1 year (3.2% vs 2.5%; absolute RD, 0.7% [95% CI, -1.3% to 2.7%]). At 1 year there was no significant difference in improvement in quality of life between the groups (difference in improvement in the Kansas City Cardiomyopathy Questionnaire overall summary score, -2.4 [95% CI, -5.1 to 0.3]; P = .08).
Conclusions and relevance: In this preliminary, registry-based study of propensity-matched patients who had undergone transcatheter aortic valve replacement for aortic stenosis, patients with bicuspid vs tricuspid aortic stenosis had no significant difference in 30-day or 1-year mortality but had increased 30-day risk for stroke. Because of the potential for selection bias and the absence of a control group treated surgically for bicuspid stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis.
Conflict of interest statement
Figures
Comment in
-
Bicuspid Aortic Valve Stenosis: Is There a Role for TAVR?JAMA. 2019 Jun 11;321(22):2170-2171. doi: 10.1001/jama.2019.7078. JAMA. 2019. PMID: 31184722 No abstract available.
-
Mortality Due to Aortic Stenosis in the United States, 2008-2017.JAMA. 2019 Jun 11;321(22):2236-2238. doi: 10.1001/jama.2019.6292. JAMA. 2019. PMID: 31184728 Free PMC article.
References
-
- Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111(7):920-925. doi:10.1161/01.CIR.0000155623.48408.C5 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

