A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses
- PMID: 31185007
- PMCID: PMC6559632
- DOI: 10.1371/journal.pbio.3000281
A novel druggable interprotomer pocket in the capsid of rhino- and enteroviruses
Abstract
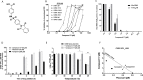
Rhino- and enteroviruses are important human pathogens, against which no antivirals are available. The best-studied inhibitors are "capsid binders" that fit in a hydrophobic pocket of the viral capsid. Employing a new class of entero-/rhinovirus inhibitors and by means of cryo-electron microscopy (EM), followed by resistance selection and reverse genetics, we discovered a hitherto unknown druggable pocket that is formed by viral proteins VP1 and VP3 and that is conserved across entero-/rhinovirus species. We propose that these inhibitors stabilize a key region of the virion, thereby preventing the conformational expansion needed for viral RNA release. A medicinal chemistry effort resulted in the identification of analogues targeting this pocket with broad-spectrum activity against Coxsackieviruses B (CVBs) and compounds with activity against enteroviruses (EV) of groups C and D, and even rhinoviruses (RV). Our findings provide novel insights in the biology of the entry of entero-/rhinoviruses and open new avenues for the design of broad-spectrum antivirals against these pathogens.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

