Government policy interventions to reduce human antimicrobial use: A systematic review and evidence map
- PMID: 31185011
- PMCID: PMC6559631
- DOI: 10.1371/journal.pmed.1002819
Government policy interventions to reduce human antimicrobial use: A systematic review and evidence map
Abstract
Background: Growing political attention to antimicrobial resistance (AMR) offers a rare opportunity for achieving meaningful action. Many governments have developed national AMR action plans, but most have not yet implemented policy interventions to reduce antimicrobial overuse. A systematic evidence map can support governments in making evidence-informed decisions about implementing programs to reduce AMR, by identifying, describing, and assessing the full range of evaluated government policy options to reduce antimicrobial use in humans.
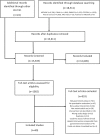
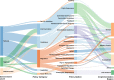
Methods and findings: Seven databases were searched from inception to January 28, 2019, (MEDLINE, CINAHL, EMBASE, PAIS Index, Cochrane Central Register of Controlled Trials, Web of Science, and PubMed). We identified studies that (1) clearly described a government policy intervention aimed at reducing human antimicrobial use, and (2) applied a quantitative design to measure the impact. We found 69 unique evaluations of government policy interventions carried out across 4 of the 6 WHO regions. These evaluations included randomized controlled trials (n = 4), non-randomized controlled trials (n = 3), controlled before-and-after designs (n = 7), interrupted time series designs (n = 25), uncontrolled before-and-after designs (n = 18), descriptive designs (n = 10), and cohort designs (n = 2). From these we identified 17 unique policy options for governments to reduce the human use of antimicrobials. Many studies evaluated public awareness campaigns (n = 17) and antimicrobial guidelines (n = 13); however, others offered different policy options such as professional regulation, restricted reimbursement, pay for performance, and prescription requirements. Identifying these policies can inform the development of future policies and evaluations in different contexts and health systems. Limitations of our study include the possible omission of unpublished initiatives, and that policies not evaluated with respect to antimicrobial use have not been captured in this review.
Conclusions: To our knowledge this is the first study to provide policy makers with synthesized evidence on specific government policy interventions addressing AMR. In the future, governments should ensure that AMR policy interventions are evaluated using rigorous study designs and that study results are published.
Protocol registration: PROSPERO CRD42017067514.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: SJH and MM have both advised several governments in their personal capacities on policy interventions that could be used to address antimicrobial resistance. The authors declare no financial relationships with any organisations that might have an interest in the submitted work.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

