Divergent roles for the RH5 complex components, CyRPA and RIPR in human-infective malaria parasites
- PMID: 31185066
- PMCID: PMC6588255
- DOI: 10.1371/journal.ppat.1007809
Divergent roles for the RH5 complex components, CyRPA and RIPR in human-infective malaria parasites
Abstract
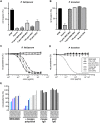
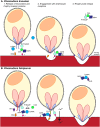
Malaria is caused by Plasmodium parasites, which invade and replicate in erythrocytes. For Plasmodium falciparum, the major cause of severe malaria in humans, a heterotrimeric complex comprised of the secreted parasite proteins, PfCyRPA, PfRIPR and PfRH5 is essential for erythrocyte invasion, mediated by the interaction between PfRH5 and erythrocyte receptor basigin (BSG). However, whilst CyRPA and RIPR are present in most Plasmodium species, RH5 is found only in the small Laverania subgenus. Existence of a complex analogous to PfRH5-PfCyRPA-PfRIPR targeting BSG, and involvement of CyRPA and RIPR in invasion, however, has not been addressed in non-Laverania parasites. Here, we establish that unlike P. falciparum, P. knowlesi and P. vivax do not universally require BSG as a host cell invasion receptor. Although we show that both PkCyRPA and PkRIPR are essential for successful invasion of erythrocytes by P. knowlesi parasites in vitro, neither protein forms a complex with each other or with an RH5-like molecule. Instead, PkRIPR is part of a different trimeric protein complex whereas PkCyRPA appears to function without other parasite binding partners. It therefore appears that in the absence of RH5, outside of the Laverania subgenus, RIPR and CyRPA have different, independent functions crucial for parasite survival.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- World Health Organization. World malaria report 2018. 2018.
-
- World Health Organization. World malaria report 2017. 2017.
-
- Weiss GE, Gilson PR, Taechalertpaisarn T, Tham W-H, de Jong NWM, Harvey KL, et al. Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Plasmodium falciparum Invasion of Erythrocytes. PLOS Pathog. 2015;11: e1004670 10.1371/journal.ppat.1004670 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

