The Efficacy of a Scaffold-free Bio 3D Conduit Developed from Autologous Dermal Fibroblasts on Peripheral Nerve Regeneration in a Canine Ulnar Nerve Injury Model: A Preclinical Proof-of-Concept Study
- PMID: 31185736
- PMCID: PMC6767885
- DOI: 10.1177/0963689719855346
The Efficacy of a Scaffold-free Bio 3D Conduit Developed from Autologous Dermal Fibroblasts on Peripheral Nerve Regeneration in a Canine Ulnar Nerve Injury Model: A Preclinical Proof-of-Concept Study
Abstract
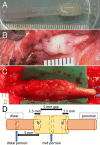
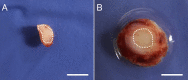
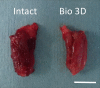
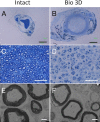
Autologous nerve grafting is widely accepted as the gold standard treatment for segmental nerve defects. To overcome the inevitable disadvantages of the original method, alternative methods such as the tubulization technique have been developed. Several studies have investigated the characteristics of an ideal nerve conduit in terms of supportive cells, scaffolds, growth factors, and vascularity. Previously, we confirmed that biological scaffold-free conduits fabricated from human dermal fibroblasts promote nerve regeneration in a rat sciatic nerve injury model. The purpose of this study is to evaluate the feasibility of biological scaffold-free conduits composed of autologous dermal fibroblasts using a large-animal model. Six male beagle dogs were used in this study. Eight weeks before surgery, dermal fibroblasts were harvested from their groin skin and grown in culture. Bio 3D conduits were assembled from proliferating dermal fibroblasts using a Bio 3D printer. The ulnar nerve in each dog's forelimb was exposed under general anesthesia and sharply cut to create a 5 mm interstump gap, which was bridged by the prepared 8 mm Bio 3D conduit. Ten weeks after surgery, nerve regeneration was investigated. Electrophysiological studies detected compound muscle action potentials (CMAPs) of the hypothenar muscles and motor nerve conduction velocity (MNCV) in all animals. Macroscopic observation showed regenerated ulnar nerves. Low-level hypothenar muscle atrophy was confirmed. Immunohistochemical, histological, and morphometric studies confirmed the existence of many myelinated axons through the Bio 3D conduit. No severe adverse event was reported. Hypothenar muscles were re-innervated by regenerated nerve fibers through the Bio 3D conduit. The scaffold-free Bio 3D conduit fabricated from autologous dermal fibroblasts is effective for nerve regeneration in a canine ulnar nerve injury model. This technology was feasible as a treatment for peripheral nerve injury and segmental nerve defects in a preclinical setting.
Keywords: Bio 3D conduit; nerve regeneration; peripheral nerve injury; preclinical study; proof of concept; scaffold-free.
Conflict of interest statement
Figures


Similar articles
-
Nerve regeneration using the Bio 3D nerve conduit fabricated with spheroids.J Artif Organs. 2022 Dec;25(4):289-297. doi: 10.1007/s10047-022-01358-9. Epub 2022 Aug 15. J Artif Organs. 2022. PMID: 35970971 Review.
-
The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model.PLoS One. 2017 Feb 13;12(2):e0171448. doi: 10.1371/journal.pone.0171448. eCollection 2017. PLoS One. 2017. PMID: 28192527 Free PMC article.
-
Efficacy and safety of Bio 3D conduits composed of human umbilical cord-derived mesenchymal stromal cells: A proof-of-concept study in a canine ulnar nerve defect model.Cell Transplant. 2025 Jan-Dec;34:9636897251361711. doi: 10.1177/09636897251361711. Epub 2025 Aug 3. Cell Transplant. 2025. PMID: 40754895 Free PMC article.
-
Bridging a 30 mm defect in the canine ulnar nerve using vessel-containing conduits with implantation of bone marrow stromal cells.Microsurgery. 2016 May;36(4):316-24. doi: 10.1002/micr.22391. Epub 2015 Mar 14. Microsurgery. 2016. PMID: 25773965
-
The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays.J Tissue Eng Regen Med. 2019 Nov;13(11):2077-2100. doi: 10.1002/term.2945. Epub 2019 Aug 20. J Tissue Eng Regen Med. 2019. PMID: 31350868 Review.
Cited by
-
Development of a common peroneal nerve injury model in domestic swine for the study of translational neuropathic pain treatments.J Neurosurg. 2021 Apr 16;135(5):1516-1523. doi: 10.3171/2020.9.JNS202961. Print 2021 Nov 1. J Neurosurg. 2021. PMID: 33862596 Free PMC article.
-
Advances in 3D printing combined with tissue engineering for nerve regeneration and repair.J Nanobiotechnology. 2025 Jan 3;23(1):5. doi: 10.1186/s12951-024-03052-9. J Nanobiotechnology. 2025. PMID: 39754257 Free PMC article. Review.
-
3D Printed Personalized Nerve Guide Conduits for Precision Repair of Peripheral Nerve Defects.Adv Sci (Weinh). 2022 Apr;9(12):e2103875. doi: 10.1002/advs.202103875. Epub 2022 Feb 18. Adv Sci (Weinh). 2022. PMID: 35182046 Free PMC article. Review.
-
Nerve regeneration using the Bio 3D nerve conduit fabricated with spheroids.J Artif Organs. 2022 Dec;25(4):289-297. doi: 10.1007/s10047-022-01358-9. Epub 2022 Aug 15. J Artif Organs. 2022. PMID: 35970971 Review.
-
Nerve regeneration using a Bio 3D conduit derived from umbilical cord-Derived mesenchymal stem cells in a rat sciatic nerve defect model.PLoS One. 2024 Dec 23;19(12):e0310711. doi: 10.1371/journal.pone.0310711. eCollection 2024. PLoS One. 2024. PMID: 39715170 Free PMC article.
References
-
- Griffin J, Hogan M, Chhabra B, Deal N. Peripheral nerve repair and reconstruction. J Bone Joint Surg Am. 2013;95(23):2144–2151. - PubMed
-
- Dahlin L. Techniques of peripheral nerve repair. Scandinavian J Surg. 2008;97(4):310–316. - PubMed
-
- Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25(4):258–267. - PubMed
-
- Mackinnon S, Doolabh V, Novak C, Trulock E. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107(6):1419–1429. - PubMed
-
- Konofaos P, Halen J. Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg. 2013;29(3):149–164. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

