Embryogenesis of flattened colonies implies the innovation required for the evolution of spheroidal colonies in volvocine green algae
- PMID: 31185890
- PMCID: PMC6560780
- DOI: 10.1186/s12862-019-1452-x
Embryogenesis of flattened colonies implies the innovation required for the evolution of spheroidal colonies in volvocine green algae
Abstract
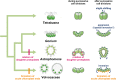
Background: Volvocine algae provide a suitable model for investigation of the evolution of multicellular organisms. Within this group, evolution of the body plan from flattened to spheroidal colonies is thought to have occurred independently in two different lineages, Volvocaceae and Astrephomene. Volvocacean species undergo inversion to form a spheroidal cell layer following successive cell divisions during embryogenesis. During inversion, the daughter protoplasts change their shape and develop acute chloroplast ends (opposite to basal bodies). By contrast, Astrephomene does not undergo inversion; rather, its daughter protoplasts rotate during successive cell divisions to form a spheroidal colony. However, the evolutionary pathways of these cellular events involved in the two tactics for formation of spheroidal colony are unclear, since the embryogenesis of extant volvocine genera with ancestral flattened colonies, such as Gonium and Tetrabaena, has not previously been investigated in detail.
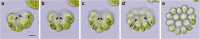
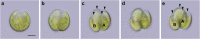
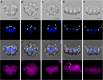
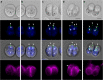
Results: We conducted time-lapse imaging by light microscopy and indirect immunofluorescence microscopy with staining of basal bodies, nuclei, and microtubules to observe embryogenesis in G. pectorale and T. socialis, which form 16-celled or 4-celled flattened colonies, respectively. In G. pectorale, a cup-shaped cell layer of the 16-celled embryo underwent gradual expansion after successive cell divisions, with the apical ends (position of basal bodies) of the square embryo's peripheral protoplasts separated from each other. In T. socialis, on the other hand, there was no apparent expansion of the daughter protoplasts in 4-celled embryos after successive cell divisions, however the two pairs of diagonally opposed daughter protoplasts shifted slightly and flattened after hatching. Neither of these two species exhibited rotation of daughter protoplasts during successive cell divisions as in Astrephomene or the formation of acute chloroplast ends of daughter protoplasts as in volvocacean inversion.
Conclusions: The present results indicate that the ancestor of Astrephomene might have newly acquired the rotation of daughter protoplasts after it diverged from the ancestor of Gonium, while the ancestor of Volvocaceae might have newly acquired the formation of acute chloroplast ends to complete inversion after divergence from the ancestor of Goniaceae (Gonium and Astrephomene).
Keywords: Body plan; Embryogenesis; Gonium; Multicellularity; Tetrabaena; Volvocine green algae.
Conflict of interest statement
Author HN is a member of the BMC Evolutionary Biology Editorial Board. The authors declare that they have no competing interests.
Figures

Similar articles
-
Alternative evolution of a spheroidal colony in volvocine algae: developmental analysis of embryogenesis in Astrephomene (Volvocales, Chlorophyta).BMC Evol Biol. 2016 Nov 9;16(1):243. doi: 10.1186/s12862-016-0794-x. BMC Evol Biol. 2016. PMID: 27829356 Free PMC article.
-
Evolution of cytokinesis-related protein localization during the emergence of multicellularity in volvocine green algae.BMC Evol Biol. 2017 Dec 6;17(1):243. doi: 10.1186/s12862-017-1091-z. BMC Evol Biol. 2017. PMID: 29212441 Free PMC article.
-
Cell size for commitment to cell division and number of successive cell divisions in multicellular volvocine green algae Tetrabaena socialis and Gonium pectorale.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(10):832-840. doi: 10.2183/pjab.93.052. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 29225309 Free PMC article.
-
Co-option during the evolution of multicellular and developmental complexity in the volvocine green algae.Curr Opin Genet Dev. 2016 Aug;39:107-115. doi: 10.1016/j.gde.2016.06.003. Epub 2016 Jul 2. Curr Opin Genet Dev. 2016. PMID: 27379901 Review.
-
Evolution of reproductive development in the volvocine algae.Sex Plant Reprod. 2011 Jun;24(2):97-112. doi: 10.1007/s00497-010-0158-4. Epub 2010 Dec 21. Sex Plant Reprod. 2011. PMID: 21174128 Free PMC article. Review.
Cited by
-
Fossil-calibrated molecular clock data enable reconstruction of steps leading to differentiated multicellularity and anisogamy in the Volvocine algae.BMC Biol. 2024 Apr 10;22(1):79. doi: 10.1186/s12915-024-01878-1. BMC Biol. 2024. PMID: 38600528 Free PMC article.
References
-
- Michod RE. On the transfer of fitness from the cell to the multicellular organism. Biol Philos. 2006;20(5):967–987. doi: 10.1007/s10539-005-9018-2. - DOI
-
- Grosberg RK, Strathmann RR. The evolution of multicellularity: a minor major transition? Annu Rev Ecol Evol Syst. 2007;38(1):621–654. doi: 10.1146/annurev.ecolsys.36.102403.114735. - DOI
-
- Nozaki H, Ito M. Phylogenetic relationships within the colonial Volvocales (Chlorophyta) inferred from cladistic analysis based on morphological data. J Phycol. 1994;30(2):353–365. doi: 10.1111/j.0022-3646.1994.00353.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

