The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis
- PMID: 31186034
- PMCID: PMC6560890
- DOI: 10.1186/s12967-019-1949-5
The expression of NOD2, NLRP3 and NLRC5 and renal injury in anti-neutrophil cytoplasmic antibody-associated vasculitis
Abstract
Background: Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are intracellular sensors of pathogens and molecules from damaged cells to regulate the inflammatory response in the innate immune system. Emerging evidences suggested a potential role of NLRs in anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV). This study aimed to investigate the expression of nucleotide-binding oligomerization domain containing protein 2 (NOD2), NOD-like receptor family pyrin domain containing 3 (NLRP3) and NOD-like receptor family CARD domain containing 5 (NLRC5) in kidneys of AAV patients, and further explored their associations with clinical and pathological parameters.
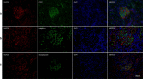
Methods: Thirty-four AAV patients in active stage were recruited. Their renal specimens were processed with immunohistochemistry to assess the expression of three NLRs, and with double immunofluorescence to detect NLRs on intrinsic and infiltrating cells. Analysis of gene expression was also adopted in cultured human podocytes. The associations between expression of NLRs and clinicopathological parameters were analyzed.
Results: The expression of NOD2, NLRP3 and NLRC5 was significantly higher in kidneys from AAV patients than those from normal controls, minimal change disease or class IV lupus nephritis. These NLRs co-localized with podocytes and infiltrating inflammatory cells. The mean optical density of NOD2 in glomeruli was significantly higher in crescentic class than non-crescentic class, and correlated with levels of proteinuria and serum creatinine at renal biopsy. The mean optical density of NLRC5 in glomeruli was significantly higher in crescentic class than non-crescentic class, and correlated with proteinuria level, Birmingham Vasculitis Activity Score and the proportion of crescents in the renal specimen.
Conclusions: The expression of three NLRs was upregulated in kidneys of AAV patients. The expression of NOD2 and NLRC5 was associated with the severity of renal lesions in AAV.
Keywords: ANCA; NOD-like receptors; Vasculitis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
- 2016YFC0906102/National Key Research and Development Program/International
- 81870478/National Natural Science Fund/International
- 81425008/National Natural Science Fund/International
- 81621092/National Natural Science Fund/International
- BMU2017CJ002/Peking University Health Science Center/International
LinkOut - more resources
Full Text Sources

