Phenotypic variation between siblings with Metachromatic Leukodystrophy
- PMID: 31186049
- PMCID: PMC6560893
- DOI: 10.1186/s13023-019-1113-6
Phenotypic variation between siblings with Metachromatic Leukodystrophy
Abstract
Background: Metachromatic Leukodystrophy (MLD) is a rare autosomal-recessive lysosomal storage disorder caused by mutations in the ARSA gene. While interventional trials often use untreated siblings as controls, the genotype-phenotype correlation is only partly understood, and the variability of the clinical course between siblings is unclear with some evidence for a discrepant clinical course in juvenile patients. The aim of this study was to systematically investigate the phenotypic variation in MLD siblings in comparison to the variability in a larger MLD cohort and to case reports published in literature.
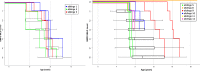
Results: Detailed clinical information was available from 12 sibling-pairs (3 late-infantile, 9 juvenile) and 61 single patients (29 late-infantile, 32 juvenile). Variability of age at onset was similar between the siblings and randomly chosen pairs of the remaining cohort (no statistically different Euclidean distances). However, in children with juvenile MLD both the type of first symptoms and the dynamic of the disease were less variable between siblings compared to the general cohort. In late-infantile patients, type of first symptoms and dynamic of disease were similarly homogeneous between siblings and the whole MLD cohort. Thirteen published case reports of families with affected siblings with MLD are presented with similar findings.
Conclusions: In a systematic analysis of phenotypic variation in families with MLD, siblings with the late-infantile form showed a similar variability as unrelated pairs of children with late-infantile MLD, whereas siblings with juvenile MLD showed a more homogeneous phenotype regarding type of first symptoms and disease evolution in comparison to unrelated children with juvenile MLD, but not regarding their age at onset. These results are highly relevant with respect to the evaluation of treatment effects and for counseling of families with affected siblings.
Keywords: Genetics; Genotype; Metachromatic leukodystrophy; Natural course; Siblings.
Conflict of interest statement
S.G. reports an institutional research grant from Shire plc, outside of the submitted work, and serves as co-investigator and advisor in a multicentre enzyme replacement trial sponsored by Shire plc, but receives no personal payment related to this role.
Figures



References
-
- Gieselmann V, Krägeloh-Mann I. Metachromatic Leukodystrophy. In: Valle D, Beaudet AL, Vogelstein B, Kinzler KW, Antonarakis SE, Ballabio A, et al., editors. The online metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2014. p. chapter 148.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

