Temporal changes within the (bladder) tumor microenvironment that accompany the therapeutic effects of the immunocytokine NHS-IL12
- PMID: 31186063
- PMCID: PMC6558846
- DOI: 10.1186/s40425-019-0620-2
Temporal changes within the (bladder) tumor microenvironment that accompany the therapeutic effects of the immunocytokine NHS-IL12
Abstract
Background: While significant strides in the treatment of metastatic bladder cancer have been made with immune checkpoint inhibitors, the treatment of carcinoma in situ and non-muscle invasive, non-metastatic (superficial) human urothelial carcinoma, also termed non-muscle invasive bladder cancer (NMIBC), remains intractable with bacillus Calmette-Guerin (BCG) employed as the standard of care. In this study, an immunocytokine, NHS-muIL12, which consists of two molecules of murine IL-12 fused to NHS76, a tumor necrosis-targeting human IgG1, was examined as an immunotherapeutic in an orthotopic MB49luc bladder tumor model.
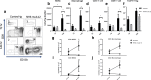
Methods: The antitumor activity of systemic administration of NHS-muIL12 was investigated on MB49luc tumors, an aggressive, bioluminescent orthotopic bladder cancer model. Temporal studies were carried out on MB49luc bladder tumors harvested during various time points during NHS-muIL12 treatment and cellular changes associated with the reduction in tumor burden following NHS-muIL12 were determined by flow cytometry. Effects of those changes on the proliferation/activation of lymphoid cells were also determined.
Results: Studies revealed a significant reduction in MB49luc bladder tumor burden occurring between days 3 and 6 after the third and final systemic administration of NHS-muIL12. Temporal analyses of the MB49luc bladder tumor microenvironment (TME) initially revealed a large accumulation of myeloid-derived suppressor cells (MDSCs) and macrophages that elicited potent immunosuppression. Immunosuppression was characterized by the inability of CD4+ and CD8+ T cells to respond to broad-based immune stimulants. NHS-muIL12 administration resulted in temporal-dependent reductions in the number of MDSCs, macrophages and tumor-associated TGF-β, which culminated in a re-ignition of CD4+ and CD8+ T cells to elicit potent antitumor responses against MB49luc bladder tumors.
Conclusions: These findings provide strong evidence that the systemic administration of an immunocytokine consisting of a tumor-targeting Ig through recognition of DNA and DNA-histone complexes coupled to muIL-12 can effectively target the bladder TME; this significantly reduces the myeloid cellular compartment and reverts an immunosuppressive to an immunopermissive TME, ultimately resulting in antitumor effects. These studies provide further rationale for the employment of NHS-IL12 as an immunomodulator and clinical immunotherapeutic for NMIBC.
Keywords: Immunotherapy; NHS-muIL12; Non-muscle invasive bladder cancer.
Conflict of interest statement
Authors from the National Cancer Institute do not have any competing interests to disclose. Author from EMD Serono is an employee/officer of said company.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
