Cell recovery by reversal of ferroptosis
- PMID: 31186229
- PMCID: PMC6602333
- DOI: 10.1242/bio.043182
Cell recovery by reversal of ferroptosis
Abstract
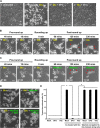
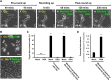
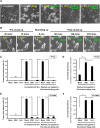
The classical view of cell death has long assumed that, once initiated, the dying process is irreversible. However, recent studies reveal that recovery of dying cells can actually occur, even after initiation of a cell suicide process called apoptosis. This discovery raised fundamental key questions about which forms of the cell death process could be reversible and how reversal is mediated. Here, we uncover an unanticipated reversibility of ferroptotic cell death process. Unlike apoptosis reversal, removal of ferroptosis inducers, such as erastin and glutamate, is insufficient to allow ferroptotic dying cells to escape the cell death process. However, by removing the cell death inducer and providing the reduced form of glutathione or the radical-trapping antioxidant ferrostatin-1, ferroptotic dying cells can be rescued and promoted to recover. Interestingly, although ferroptotic inhibitors such as aminooxyacetic acid, deferoxamine, dopamine and vitamin C can prevent initiation of ferroptosis, added alone they are unable to reverse the initiated ferroptosis, suggesting regulatory distinctions between preventing and reversing ferroptosis. Together, these results reveal the first evidence that ferroptosis is reversible and suggest strategies to enhance its reversibility, thereby providing a useful model for studying the physiological, pathological and therapeutic potentials of this cell recovery process.
Keywords: Anastasis; Ferrostatin-1; Glutamate; Glutathione; Reversal of apoptosis; Reversal of ferroptosis.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K. and Walter P. (2002). The cell cycle and programmed cell death. In Molecular Biology of The Cell, pp. 983-1026, 4th edn New York, NY: Garland Science, Taylor and Francis Group.
-
- Alberts B., Johnson A., Lewis J., Morgan D., Raff M., Roberts K. and Walter P. (2014). Cell death. In Molecular Biology of The Cell, pp. 1021-1034, 6th edn New York, NY: Garland Science, Taylor and Francis Group.
Grants and funding
LinkOut - more resources
Full Text Sources

